Figure 7.
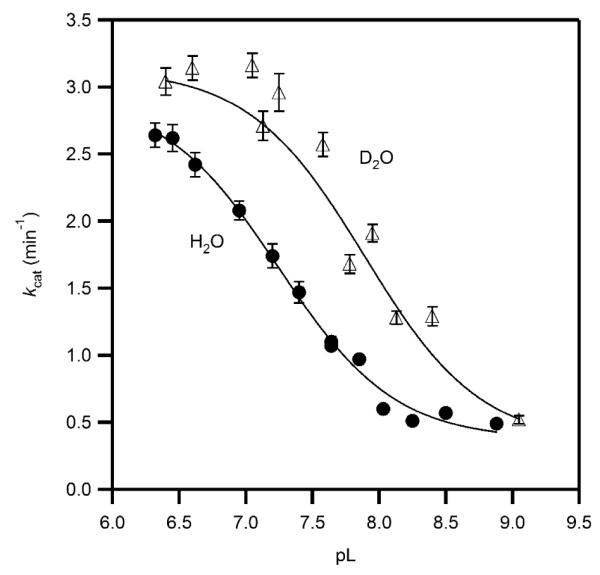
Solvent isotope effect on the apparent kcat for PHD2 in MPH buffer at 37.0 °C, ambient [O2] (D2O, open triangles, H2O, closed circles), fitted to pH-dependent model. The acid form of PHD2 exhibited kcat = 2.99(8) min−1 in H2O, and 3.30(6) min−1 in D2O, for D2Okcat = 0.91(3). The base form of PHD2 exhibited kcat = 0.31(2) min−1 in H2O, and 0.34(4) min−1 in D2O, for D2Okcat = 0.9(1). The pKa = 7.22(3) in H2O; pKa = 7.89(3) in D2O.
