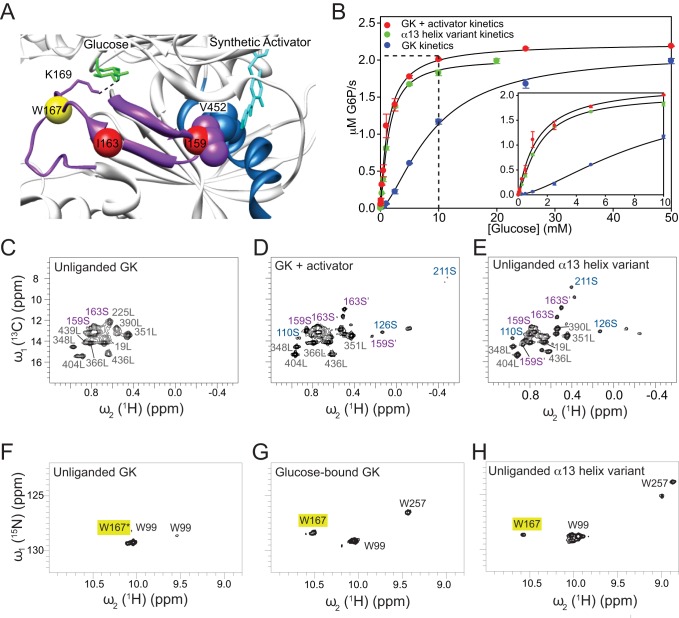
Figure 2. Structural, dynamic, and kinetic descriptions of GCK activating states.
(A) Crystal structure of the GCK–activator complex depicting the spatial vicinity of the regions involved in allosteric communication. The 151–179 β-hairpin is shown in magenta, the α13-helix in blue, and the Cαs of Ile-159, Ile-163, and Trp-167 residues are colored in red (Ile) and yellow (Trp) spheres. Glucose is colored in green, and the synthetic allosteric activator in cyan. The side chain of Lys-169, which is represented by magenta sticks, is hydrogen-bonded to O6 of glucose. Val-452 and Ile-159 side chains are located within 5 Å of each other and of the activator thiazole ring. (B) Kinetic response of the α13-helix variant (green), and wild-type GCK in the absence (blue) and presence (red) of saturating activator (see Table S1). 2D 1H-13C HMQC spectra of (C) unliganded GCK, (D) activator-bound complex, and (E) unliganded α13-helix variant, specifically labeled with Ile 13C(δ1) methyl groups. 2D 1H-15N HSQC spectra of (F) unliganded GCK, (G) glucose-bound complex, and (H) unliganded α13-helix variant, with Trp-15Nε cross-peaks assigned by black labels. The yellow box highlights the position of the W167 cross-peak. The asterisk indicates that W167 is the main contributor to the intensity of the unliganded cross-peak (for more details, see Figure S3).