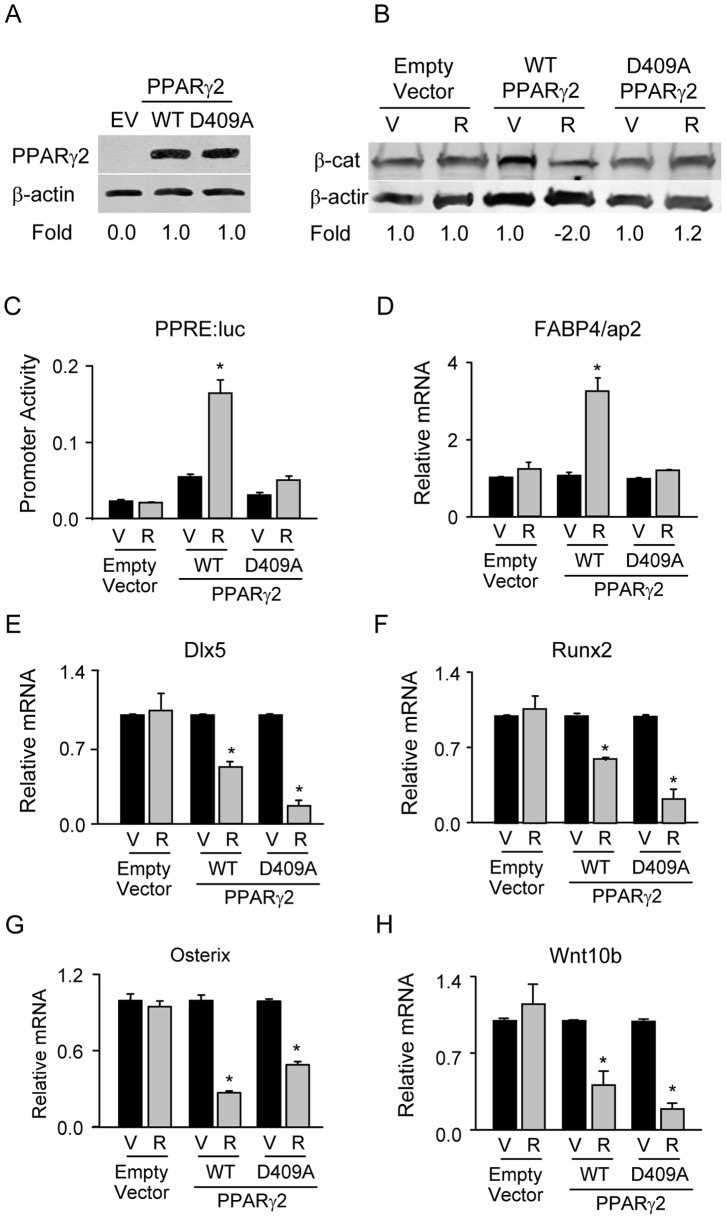
Figure 6. PPARγ2 mutation, abrogating the pro-adipocytic but not the anti-osteoblastic activity, protects β-catenin protein from degradation.
A. Western blot analysis of protein levels of non-mutated (WT) and mutated (D409A) forms of PPARγ2 analyzed 72 h after transfection of HEK293 cells. β-actin was used as a loading control. Each lane was loaded with 50 µg of total protein lysate. EV – empty vector control. B. Western blot analysis of β-catenin protein levels after treatment with 1 µM Rosi for 72 h. Hek293 cells were transfected with β-catenin expression construct and either empty expression vectors (pSPORT6 and pEF-BOS), or non-mutated (WT), or mutated (D409A) PPARγ2 expression constructs. Each lane was loaded with 50 µg of total protein lysate. C. Effect of D409A mutation on transcriptional activity of PPARγ2. Hek293 cells were transiently transfected with above constructs and co-transfected with p2AOx luciferase reporter gene construct under the control PPARγ response elements. Cells were treated with either vehicle or 1 µM Rosi for 48 h and lysates were analyzed for luciferase activity. Promoter activity of firefly luciferase was normalized to renilla luciferase which was used as a transfection control. D – G. Effect of D409A mutation on expression of adipocyte-specific (D) and osteoblast-specific (E – G) gene markers, and Wnt10b (H). U-33/c cells were transiently transfected with either empty vector (pEF-BOS), or non-mutated (WT), or mutated (D409A) PPARγ2 expression constructs and treated with either vehicle or 1 µM Rosi for 72 h. Relative transcript levels were calculated as fold change as compared to vehicle treated cells in each transfection. V – vehicle; R- Rosi; * p<0.05 V vs. R.