Abstract
Tumors employ various mechanisms to escape elimination by the immune system. In addition to the local induction of immunosuppressive cell types such as regulatory T cells or myeloid derived suppressor cells, tumor antigen shedding into the circulation may suppress antitumor CD8+ T-cell function via tolerogenic liver sinusoidal endothelial cells.
Keywords: B7H1; CD8+ T cells; CEA; circulating antigen; liver sinusoidal endothelial cells; tumor immune escape,
Tumors develop in constant interaction with the immune system, which influences both oncogenesis and tumor progression. This process has been conceptualized by the theory of cancer immunoediting, which involves an elimination, an equilibrium and an escape phase.1 During cancer immunoediting, tumors eventually develop ways to escape efficient antitumor immunity. One such ways is to create immunomodulatory conditions within the tumor microenvironment.2 The local production of immunosuppressive factors such as transforming growth factor β (TGFβ) and interleukin-10 (IL-10), the induction of enzymes that consume essential nutrients for immune cell function (e.g., IDO, Arg-I), and the local activation or recruitment of immunosuppressive cell types (e.g., regulatory T cells, myeloid derived suppressor cells) can all contribute to limit immune responses.
In many clinical settings, the presence of tumor-associated antigens in the serum of patients is being used for diagnostic and sometimes prognostic purposes. For instance, in colorectal carcinoma (CRC) patients, the levels of the carcinoembryonic antigen (CEA) in the serum has clinical relevance3 and is used as a prognostic maker. We hypothesized that the release of such antigens by tumor cells into the bloodstream might influence antitumor immunity. This strategy would add up to local immunosuppressive mechanisms engaged by tumor cells following the interaction with immune cells in their close proximity.
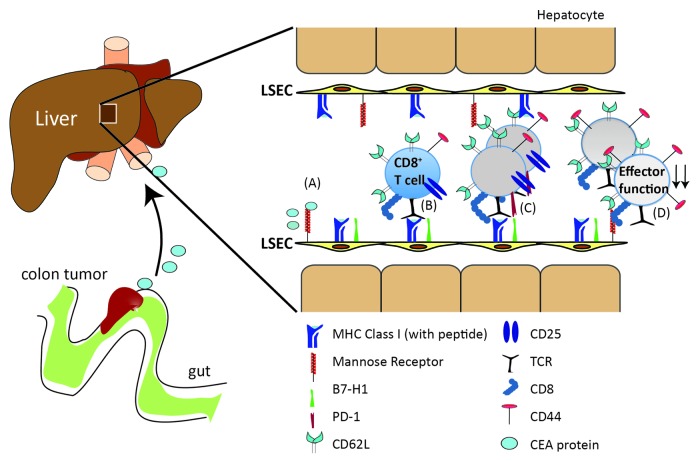
The first questions that arise when tumor antigens gain access to circulation are which antigen-presenting cells take them up and to which immune cells do these antigens get presented. Splenic and hepatic myeloid and dendritic cells, both of which are known to filter blood antigens, were not involved in the uptake of circulating CEA or its cross-presentation to CEA-specific CD8+ T cells in mice.4 Conversely, liver sinusoidal endothelial cells (LSEC) were the major population scavenging and cross-presenting CEA to CEA-specific CD8+ T cells in a mannose receptor dependent fashion.4 Recognition of cross-presented CEA led to the induction of an expanded population of functionally incapacitated CD8+ T cells with a distinct phenotype, being CD44highCD62LhighCD25neg and hence differing from both naïve and effector CD8+ T cells.4 These LSEC-stimulated CD8+ T cells were neither able to produce cytokines, nor were they cytotoxic, resulting in their inability to control the growth of CEA-expressing tumors (Fig. 1). Thus, by shedding antigens into the circulation, tumors have another means of activating immunosuppressive mechanisms.
Figure 1. (A) The tumor carcinoembryonic antigen (CEA) circulating in the blood stream can be taken up by the mannose receptor (MR) and cross-presented by liver sinusoidal endothelial cells (LSECs). (B, C) Antigen recognition by naive CD8+ T cells on LSECs leads to proliferation and expansion of CD8+ T cells and the emergence of an antigen-experienced CD8+ T-cell population that has upregulated CD44 but is CD25- and remains CD62Lhigh. (D) LSEC-induced CD8+ T cells are retained in the T-cell repertoire but are unable to produce interleukin-2 (IL-2) and interferon γ (IFNγ) and hence cannot mount a cytotoxic response to antigen-bearing target cells. As a consequence, CEA-specific LSEC-primed CD8+ T cells are unable to eradicate tumor cells in vivo. The induction of such incapacitated CD8+ T cells by LSECs is dependent on expression of the co-inhibitory molecule B7H1.
Interestingly, we could detect a similar phenotypically and functionally incapacitated CD8+ T cell population among peripheral blood mononuclear cells (PBMCs) from healthy individuals, indicating that—in general—circulating antigens, for instance food antigens, can induce such tolerant CD8+ T cells in humans as well. In CRC patients, the frequency of this cell population was increased and contained CD8+ T cells specific for the tumor-associated protein CEA,4 indicating that the induction of non-functional tumor-specific CD8+ T cells as a result of antigen recognition on LSECs also occurs in humans. In mice, we found that the expression of the co-inhibitory molecule B7H1 on LSECs was essential for the inhibition of effector function in tumor-specific CD8+ T cells.4,5 Blocking B7H1/PD-1 interactions can restore the function of exhausted PD-1+ CD8+ T cells in chronic viral infections6 and B7H1/PD-1-blocking antibodies are currently being tested in cancer patients.7,8 Although antigen-specific CD8+ T cells that are generated after systemic antigen distribution do not express PD-1, we would expect that the induction of new incapacitated cells may well be prevented by blocking B7H1/PD-1 interactions, resulting in the generation of CD8+ T cells with full anti-tumor effector potential.
Using bone marrow chimeras in which antigen presentation to CD8+ T cells was restricted to LSECs and CD8+ T cells bearing congenic markers, facilitating the tracking of antigen-specific T cells in vivo, we have defined LSEC-induced CD8+ T cells to be a CD44highCD62LhighCD25neg CD8+ T-cell subset.4,5 In addition to their impaired effector function, the high expression of CD62L may lead to the redistribution of these CD8+ T cells to lymphoid organs that are distant from the tumor site. This particular marker expression profile sets these cells apart from naïve (CD44low) and effector (CD25+CD62Llow) CD8+ T cells, but not memory T cells. Therefore, the identification of a specific marker for LSEC-induced CD8+ T cells is awaited to make the study of these cells in humans more straightforward.
We have found that the distribution of tumor antigens in the circulation can inhibit CD8+ T cell-mediated antitumor immunity via the presentation of antigens on LSECs,4,5 which strengthens the notion indicating the liver as a tolerogenic organ.9 Moreover, presentation of circulating antigens by LSECs may have implications for the CD4+ T- cell repertoire, as LSECs have been reported to induce a population of FOXP3- CD4+ T cells capable of suppressing autoimmune hepatitis.10 In this way, tumor cells shedding their antigens into the bloodstream may not only directly diminish antitumor CD8+ T-cell responses, but also may be able to modulate antitumor CD4+ T cell responses. As many tumor-associated antigens can be detected in the circulation of cancer patients (e.g., α-fetoprotein, CA-19–9 or CA-125), we postulate that the inhibition of antitumor CD8+ T-cell immunity via antigen cross-presentation by LSECs might represent a general mechanism of tumor immune escape that operates alongside immunosuppressive mechanisms within the tumor microenvironment.
Glossary
Abbreviations:
- CEA
carcinoembryonic antigen
- LSECs
liver sinusoidal endothelial cells
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21514
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338–51. doi: 10.1081/CNV-58878. [DOI] [PubMed] [Google Scholar]
- 4.Höchst B, Schildberg FA, Böttcher J, Metzger C, Huss S, Türler A, et al. Liver sinusoidal endothelial cells contribute to CD8 T cell tolerance towards circulating carcinoembryonic antigen in mice. Hepatology. 2012;••• doi: 10.1002/hep.25844. [DOI] [PubMed] [Google Scholar]
- 5.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 6.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–66. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 10.Kruse N, Neumann K, Schrage A, Derkow K, Schott E, Erben U, et al. Priming of CD4+ T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3- regulatory T cells suppressing autoimmune hepatitis. Hepatology. 2009;50:1904–13. doi: 10.1002/hep.23191. [DOI] [PubMed] [Google Scholar]