Abstract
The combination of a single low dose of cyclophosphamide (Cy) with the adenovirus-mediated gene transfer of interleukin-12 (AdIL-12) might represent a successful therapy for experimental gastrointestinal tumors. This approach has been proven to revert immunosuppressive mechanisms elicited by cancer cells and to synergistically promote antitumor immunity. In addition, this therapeutic regimen has been shown to be more efficient in achieving complete tumor regressions in mice than the application of a metronomic schedule of Cy plus AdIL-12.
Keywords: advanced gastrointestinal carcinomas, cyclophosphamide, gene therapy, immunomodulation, interleukin-12
During the last two decades, a number of immunotherapy-based strategies for advanced gastrointestinal carcinomas (GICs) has given promising results, not only at preclinical stage but also in the clinic.1 However, immunotherapeutic approaches have to face an important obstacle: the ability of tumor cells to evade immune attack.2 Several immunosuppressive mechanisms elicited by cancer cells have been identified in animal models and in patients including:1 loss of MHC Class I molecules from the surface of tumor cells,2 increased oxidative stress and3 recruitment of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs). A correlation between increased levels of these immunosuppressive cell populations and poor prognosis has been observed in many types of cancer.3
Increasing evidence suggests that immune responses are involved in the control of cancer and that the immune system can be manipulated in different ways to recognize and fight cancer cells. The systemic administration of cytokines such as interleukin (IL)-12, IL-15, granulocyte macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor α (TNFα) has been shown to be efficient in generating immunity against many tumors, although it is frequently associated with toxicity.4 We have recently demonstrated that a combination of IL-12, a potent antitumor cytokine and a single low dose of cyclophosphamide (Cy Mo) synergize with sub-therapeutic doses of an adenovirus expressing IL-12 (AdIL-12) to generate an immune response that is able to eradicate established tumor nodules. Such a combination regimen was found to mediate powerful antitumor effects against subcutaneous colorectal carcinoma (CRC), metastatic CRC and pancreatic cancer in mice.5
The combination of Cy Mo and AdIL-12 seems to have a great impact on the immunosuppressive tumor microenvironment, resulting in the depletion of Tregs. Consistently, the adoptive transfer of Tregs significantly abolished the antitumoral effects achieved by Cy Mo and AdIL-12. Several mechanisms have been proposed to explain the immunosuppressive activity Tregs, including secretion of specific cytokines and the induction of apoptosis in effector T cells. 6 We found that the combination of Cy Mo and AdIL-12 significantly reduced the production of IL-10 and transforming growth factor β (TGFβ) by Tregs and to abolish their capacity to inhibit lymphocyte proliferation. Thus, the depletion and/or inhibition of Tregs function appears to be the main immunoregulatory mechanism associated with the antitumor effects of Cy Mo and AdIL-12.5 Myeloid cells with immunosuppressive functions, including MDSCs or tumor-associated macrophages (TAM), have recently received much attention in the field of tumor immunology, as they emerged as potent suppressors of T cell-mediated immunity with a wide repertoire of effector mechanisms.7 The combination of Cy Mo and AdIL-12 also reduced the number of MDSCs in the spleen of tumor-bearing mice, hence representing a valuable strategy to impact on cell populations critically involved in tumor-induced immunosuppression.
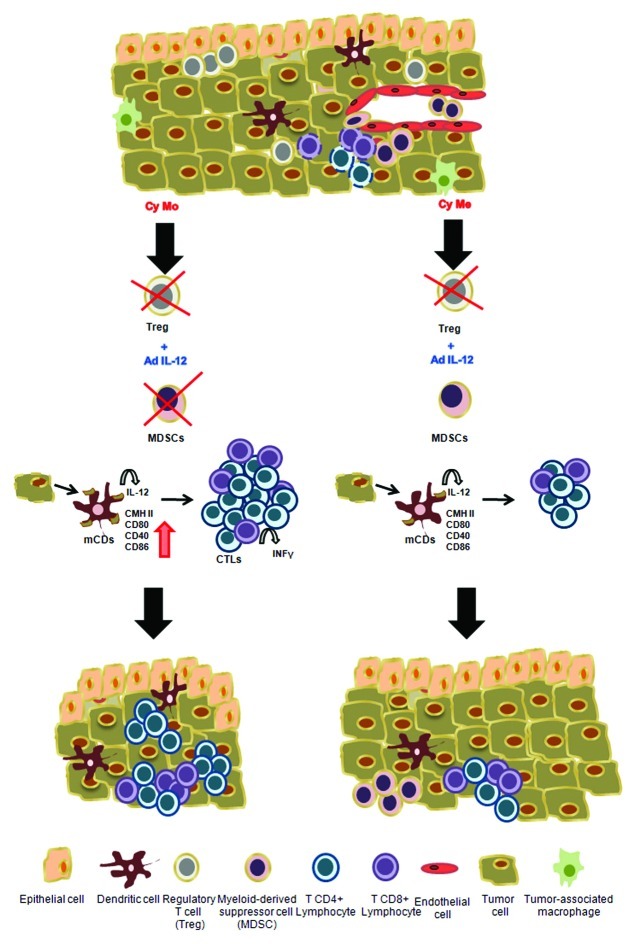
Successful immunotherapies not only reverse immunosuppressive mechanisms elicited along with tumor progression but also induce a strong cytotoxic immune response against cancer cells. The induction of antitumor immunity requires the active participation of antigen-presenting cells such as dendritic cells (DCs), which are essential for the optimal activation of T-cell responses against cancer. The use of Cy Mo contributed to increase tumor immunogenicity and to the generation of specific CTL responses by altering the maturation and/or functional status of DCs. This activity has been shown to potentiate the efficacy of gene therapy with AdIL-12.8 In addition, Cy Mo plus AdIL-12 induces the expansion of interferon γ (IFNγ)-secreting CD4+ T lymphocytes with specific cytotoxic activity against CRC cells 5 and promotes a strong tumor infiltration by CD4+ and CD8+ T cells (Fig. 1).
Figure 1. Proposed mechanisms of action underlying tumor regression as induced by the combination of monodose cyclophosphamide (Cy Mo) and IL12 gene transfer. Factors secreted by the tumor induce the mobilization of cells with immunosuppressive activity such as Tregs, myeloid-derived suppressor cells (MDSCs) and tolerogenic dendritic cells (iDCs). These cells can kill tumor-specific T cells or inhibit their activity by inducing a state of anergy. The sequential administration of single dose (Cy Mo) or metronomic dose of Cy (Cy Me) followed by IL12 gene transfer (AdIL-12) releases these brakes. However, after several weeks of treatment, Cy Me plus AdIL-12 is not as efficient as Cy Mo plus AdIL-12 in maintaining MDSCs to low levels. On the other hand, Cy Mo might promote the immunogenicity of cancer cells by improving the activation and/or maturation of antigen presenting cells. In addition, Cy Mo plus AdIL-12 stimulate the infiltration of CD4+ and CD8+ T lymphocytes while Cy Me fails not only to generate a beneficial tumor microenvironment but also to induce tumor-specific immune responses.
Previous studies have shown that Cy might potentiate the antitumor efficacy of a number of immunotherapeutic strategies. The immunomodulatory effects of Cy include a Th2/Th1 cytokine shift, the stimulation of innate immune responses, the activation of DCs and the elimination or inhibition of Tregs. Although the mechanisms of action of chemotherapeutic agents are not fully understood, it has recently been demonstrated that autophagy in cancer cells is necessary for immunogenic cell death and hence underlies optimal therapeutic effects.9 Our in vivo models suggest that some of these mechanisms are involved in the antitumor effects of Cy Mo plus AdIL-12.
The application of a Cy metronomic schedule (frequent and homogeneously spaced low-dose administrations) has been shown to induce immunostimulatory and antiangiogenic effects, opening up new avenues for cancer immunotherapy combinations.10 We therefore tested metronomic Cy (Cy Me) schemes, looking for a possible boosting in the efficacy of AdIL-12. However, this protocol was less effective than that with the combination of Cy Mo and AdIL-12. Indeed, although Cy Me plus AdIL-12 was able to decrease the incidence of Tregs and (initially) MDSCs similar to Cy Mo plus AdIL-12, it failed to maintain such an inhibition over time. Therefore, the combination of Cy Me and AdIL-12 was not as efficient as that of Cy Mo and AdIL-12 in eliminating MDSCs. In addition, this therapeutic approach failed to induce a powerful maturation of DCs and to generate a strong and specific response against CRC cells (Fig. 1). These observations might have significant implications for future strategies based on the use of Cy as an immunomodulatory adjuvant including DC-based vaccines.
The possibility of manipulating the immune system against cancer would be absolutely thrilling, and combination approaches that both stimulate potent antitumor responses and inhibit immunosubversion mechanisms elicited by cancer cells could be of paramount importance. In this regard, the combination of Cy Mo and AdIL-12 perhaps represents a good strategy to improve the immune recognition of tumor cells and, hence, to achieve an optimal therapeutic response that eradicates GICs.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21651
References
- 1.Elkord E, Hawkins RE, Stern PL. Immunotherapy for gastrointestinal cancer: current status and strategies for improving efficacy. Expert Opin Biol Ther. 2008;8:385–95. doi: 10.1517/14712598.8.4.385. [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 3.Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60:1161–71. doi: 10.1007/s00262-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 5.Malvicini M, Ingolotti M, Piccioni F, Garcia M, Bayo J, Atorrasagasti C, et al. Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12. Mol Oncol. 2011;5:242–55. doi: 10.1016/j.molonc.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malvicini M, Alaniz L. Single low-dose cyclophosphamide combined with interleukin-12 gene therapy is superior to a metronomic schedule in inducing immunity against colorectal carcinoma in mice. OncoImmunology. 2012;1:1–10. doi: 10.4161/onci.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 10.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–46. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]