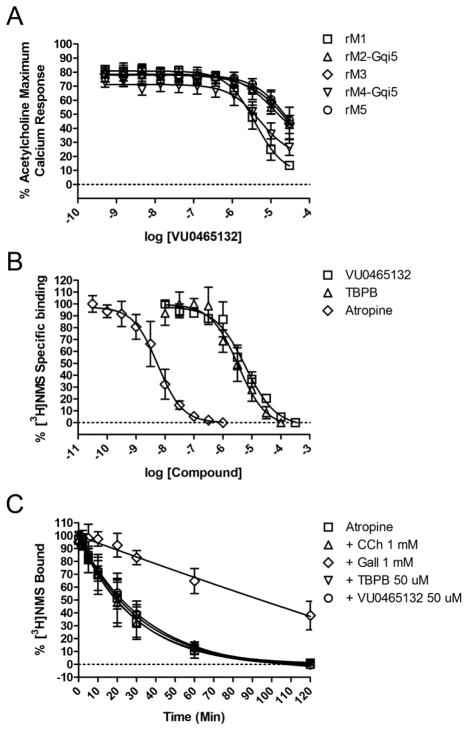
Figure 3.
Pharmacological profile of β-F-TBPB 9 (VU0465132). A) Rat M1-M5 CRCs screened as antagonists. Rat M1 IC50 = 4.9 μM (pIC50 = 5.31±0.17), 13.6±1.5% ACh Max, M2, M3 and M5 IC50 > 10 μM (pIC50s <5), M4 IC50 = 5.2 μM (pIC50 = 5.29±0.09), 26.6±3.4% ACh Max (Human M1-M5 data not shown, but comparable). B) [3H]-NMS competition binding studies. At higher concentrations than the control atropine (Ki = 1.4 nM, pKi = 8.86±0.24), both 9 (Ki = 1.35 μM, pKi = 5.87±0.18) and TBPB (Ki = 0.60 μM, pKi = 6.22±0.14) fully displace [3H]-NMS, consistent with interactions at the orthosteric ACh site. C) Dissociation kinetics with TBPB, 9, atropine, carbachol (CCh) and Gallamine (Gall). In our assays, only the allosteric modulator Gall altered the rate of [3H]-NMS dissociation, providing further evidence that 9 acts as an orthosteric antagonist. Koff rates were as follows ± SEM: Atropine (0.0379±0.0125 min−1), CCh(0.0393±0.0125 min−1), Gall (0.0012±0.0006 min−1), TBPB (0.0335±0.0095 min−1), VU0465132 (0.0308±0.0103 min−1).