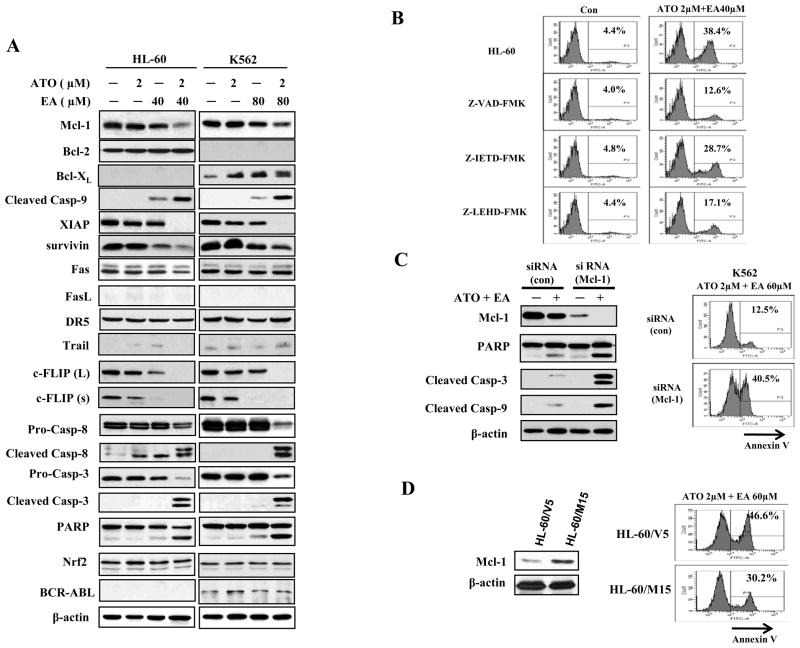
Figure 2. ATO and EA combination induces activation of caspase-3, caspase-8, and caspase-9 and dowregulates antiapoptotic proteins.
(A) Modulation of apoptotic proteins by ATO/EA. HL-60 and K562 cells were treated with EA and ATO at the indicated concentrations for 24 h. The relative levels of indicated proteins were determined by Western blotting using specific antibodies. β-actin served as loading control. (B) Caspase 9 (but not caspase 8) is the major effector of ATO/E-induced apoptosis. HL-60 cells were pretreated for 4 h with 50 μM Z-VAD-FMK (a general-caspase inhibitor), 50 μM Z-IETD-FMK (a caspase-8 inhibitor), 50 μM Z-LEHD-FMK (a caspase-9 inhibitor), and then incubated with 2 μM ATO and 40 μM EA for another 24 h. Apoptotic cells were quantified using annexin V-FITC staining and FACS analysis. Con.-control. (C) Silencing of Mcl-1 enhances ATO/EA-induced apoptosis in K562 cells. K562 cells were transfected with Mcl-1siRNAor negative control siRNA and after 18 h, treated with 2 μM ATO and 60 μM EA for additional 24 h. The protein levels were determined by Western blotting and the apoptotic cells were determined by FACS after staining with annexin V-FITC. (D) Increased Mcl-1 level attenuated ATO/EA-induced apoptosis in HL-60 cells. HL-60 cells transfected with an empty vector (HL-60/V5) and Mcl-1 expression plasmid (HL-60/M15) were treated with 2 μM ATO and 60 μM EA for additional 24 h. The Mcl-1 level was determined by Western blotting and the apoptotic cells were determined by FACS after staining with annexin V-FITC.