SUMMARY
Rationale
Multimodal imaging has the potential to identify acute ischemic stroke patients most likely to benefit from late recanalization therapies.
Aims
The general aim of the MR RESCUE Trial is to investigate whether multimodal imaging can identify patients who will benefit substantially from mechanical embolectomy for the treatment of acute ischemic stroke up to 8 hours from symptom onset.
Design
MR RESCUE is a randomized, controlled, blinded-outcome clinical trial.
Population Studied
Acute ischemic stroke patients with large vessel intracranial internal carotid artery (ICA) or middle cerebral artery (MCA) M1 or M2 occlusion enrolled within 8 hours of symptom onset are eligible. The study sample size is 120 patients.
Study Intervention
Patients are randomized to endovascular embolectomy employing the Merci Retriever (Concentric Medical, Mountain View, California) or the Penumbra System (Penumbra, Alameda, California) vs. standard medical care, with randomization stratified by penumbral pattern.
Outcomes
The primary aim of the trial is to test the hypothesis that the presence of substantial ischemic penumbral tissue visualized on multimodal imaging (MRI or CT) predicts patients most likely to respond to mechanical embolectomy for treatment of acute ischemic stroke due to a large vessel, intracranial occlusion up to 8 hours from symptom onset. This hypothesis will be tested by analyzing whether pretreatment imaging pattern has a significant interaction with treatment as a determinant of functional outcome based on the distribution of scores on the modified Rankin Scale (mRS) measure of global disability assessed 90 days post-stroke. Nested hypotheses test for 1) treatment efficacy in patients with a penumbral pattern pretreatment, and 2) absence of treatment benefit (equivalency) in patients without a penumbral pattern pretreatment. An additional aim will only be tested if the primary hypothesis of an interaction is negative: that patients treated with mechanical embolectomy have improved functional outcome vs. standard medical management.
Keywords: ischemic stroke, MRI, CT, DWI, embolectomy, penumbra, clinical trial, neuroimaging
INTRODUCTION
Salvage of the ischemic penumbra has formed the theoretical basis of recanalization therapies designed to reverse or minimize the effects of acute ischemic stroke.(1) The pivotal National Institutes of Neurological Disorders and Stroke (NINDS) trials demonstrated the clinical efficacy of intravenous (IV) thrombolysis for treatment of ischemic stroke within 3 hours of symptom onset.(2) In this hyperacute time window, advanced imaging is not needed, as it is presumed that the majority of stroke patients still have salvageable penumbral tissue. Subsequent trials and pooled analyses of IV tissue plasminogen activator (tPA) trials further demonstrated efficacy and safety in an extended time window up to 4 ½ hours employing only non-contrast brain CT imaging for screening.(3–5) However, in many communities, only 2–5% of acute stroke patients receive IV tPA, largely due to presentation beyond the first few hours of symptom onset.(6, 7) It has long been postulated that a significant number of patients still have substantial penumbral tissue at later time windows, and that advanced multimodal imaging could be employed to identify these patients and further extend the time window for acute stroke therapies.(8, 9)
Yet, to date, the penumbral selection hypothesis has not been definitively proven. Several studies, including the Diffusion and perfusion imaging Evaluation For Understanding Stroke Evolution (DEFUSE)(10) and the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET)(11) trials, have provided promising supportive evidence, but have also highlighted some of the early potential pitfalls in employing the diffusion-perfusion mismatch model for defining the core and penumbra.(12) Conclusive demonstration of the penumbral selection hypothesis ultimately requires a randomized, controlled trial with sufficient numbers of patients with penumbral and non-penumbral patterns to demonstrate that patients with both a penumbral pattern and recanalization therapy have a better clinical outcome compared to all other groups.
Endovascular interventions offer promise for the treatment of acute ischemic stroke for patients under 3–4.5 hours from onset in combination with tPA(13) or in whom conventional intravenous thrombolysis fails or is contraindicated, and for patients presenting beyond the 3–4.5 hour time window. Several trials have demonstrated technical efficacy (recanalization) with mechanical devices, and improved outcomes with achievement of recanalization.(14–16) However, these studies did not include a control arm and thus were unable to demonstrate improved outcomes compared to no treatment (or standard medical care). Ultimately, clinical efficacy (improved outcomes in the endovascular group) can only be demonstrated with randomized, controlled trials.
The MR RESCUE trial is designed specifically to test the hypothesis that patients with substantial salvageable penumbral tissue identified on multimodal imaging and treated with mechanical embolectomy will have improved outcomes compared to both untreated patients and those without penumbral tissue. Not only is MR RESCUE currently the only randomized, controlled trial designed specifically to prove the penumbral selection hypothesis for mechanical embolectomy, it will also be the first completed, randomized, controlled trial of mechanical embolectomy vs. standard medical care. Of note, MR RESCUE uniquely employs multivariate models (incorporating information from multiple types of sequences or images) for both CT and MRI to identify penumbral tissue on a voxel-by-voxel basis rather than MRI diffusion-perfusion mismatch or CT perfusion measures of mismatch.
METHODS
Design
The MR RESCUE trial is a multicenter, randomized, controlled, blinded-outcome evaluator trial of mechanical embolectomy versus standard medical care in patients experiencing acute ischemic stroke due to a proximal large vessel anterior circulation occlusion 0–8 hours from symptom onset. A total of 120 patients are being randomized 1:1 to embolectomy vs. conventional medical care, with randomization stratified by imaging pattern (penumbral vs. non-penumbral). All patients undergo pretreatment and day 7 multimodal CT or multimodal MRI imaging.
The primary goals of MR RESCUE are 1) to demonstrate that presence of substantial penumbral tissue on multimodal MR or CT predicts patients most likely to respond to mechanical embolectomy; and 2) to demonstrate that embolectomy-treated patients have improved functional outcome compared to randomized control patients. The trial began enrolling in June of 2004 with FDA approval to include 30 active sites.
Patient Population – Inclusion and Exclusion Criteria
Adult male or female patients, presenting within 8 hours of symptoms onset and meeting eligibility criteria at each of the study sites are assessed for possible enrollment into the study. Prior to enrollment, all patients undergo multimodal imaging with CT or MRI. Eligibility criteria are provided in Table 1. All sites are required to have institutional review board (IRB) approval to participate in the trial. One site is approved locally and by the Food and Drug Administration (FDA) to enroll under waiver of consent, while the remaining sites enroll via signed informed consent by the patient or patient’s legally authorized representative.
Table 1.
Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| New focal disabling neurologic deficit consistent with acute cerebral ischemia (NIHSS ≥ 6 with at least six points attributed to current stroke) | NIHSS ≥ 30 |
| Age ≥ 18 ≤ 85 | Acute intracranial hemorrhage |
| Clot retrieval procedure can be initiated within 8 hours from onset | Coma |
| Large vessel proximal anterior circulation occlusion on MR or CT angiography (internal carotid, M1 or M2 MCA) | Rapidly improving neurological signs prior to randomization |
| Signed informed consent obtained from the patient or patient’s legally authorized representative (or via waiver of consent if IRB/FDA approved for enrollment under waiver) | Pre-existing medical, neurological or psychiatric disease that would confound the neurological, functional, or imaging evaluations |
| Pretreatment multimodal MRI or CT performed according to MR RESCUE protocol | Pregnancy |
| Premorbid modified Rankin score of 0–2 | Known allergy to iodine previously refractory to pretreatment medications |
| Allowed but not required: patients treated with IV tPA up to 4.5 hours from symptom onset with persistent target occlusion on post-treatment MR RESCUE MR or CT protocol performed at the completion of drug infusion | Current participation in another experimental treatment protocol |
| Contrast-Enhanced Neck MRA or CTA suggests proximal ICA occlusion, proximal carotid stenosis > 67%, or dissection | |
| INR > 3.0 | |
| PTT > 3 × Normal | |
| Imaging data cannot be processed by MR RESCUE computer | |
| Renal failure: serum creatinine > 2.0 or GFR < 30 | |
| MRI*: contraindication to MRI | |
| CT*: contraindication to iodinated contrast |
Baseline imaging modality
Randomization
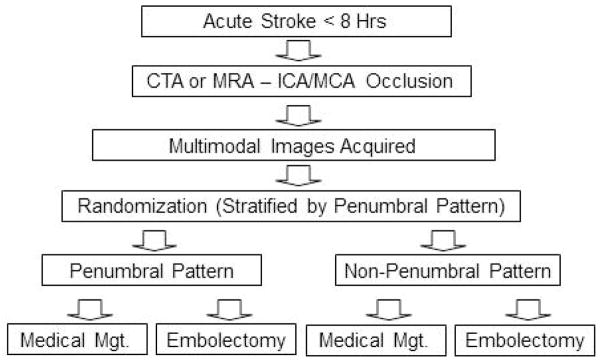
A study design flow chart is provided in Figure 1. Once inclusion/exclusion criteria are met, the imaging data are transferred to a dedicated on-site MR RESCUE computer via local area network for image post-processing. The “RescueOnSite” program requires only that the user enter which brain hemisphere is ischemic and the total NIHSS score. “RescueOnSite” image processing software operating on the dedicated computer then automatically analyzes and classifies the penumbral pattern in each patient at the time of patient presentation (assignment of each patient to the penumbral or non-penumbral group). “RescueOnSite” data processing takes approximately 3–5 minutes to perform. The automated image outputs of the “RescueOnSite” program are not displayed on the local site screen. Instead, the program displays a 4 digit code for the site to enter into the randomization web site (the code is encrypted to prevent the local investigators from knowing the whether the software has classified the results as penumbral or non-penumbral). The randomization web site recognizes the code as indicating penumbral or non-penumbral pattern and uses this information in generating the treatment assignment, with the randomization stratified by penumbral pattern and imaging modality employing a biased coin technique (weighted randomization to provide balanced assignments while maintaining uncertainty regarding next allocation.) If the post-processing fails for any reason, the “RescueOnSite” software generates a different 4 digit code indicating penumbral status is unknown, and the patient is randomized using an unknown permuted block sequence separate from the stratified permuted block sequences, to allow randomization without further delay. These cases are later post-processed at the central neuroimaging coordinating center, assigned to the correct pattern category, and this information is used in weighting of subsequent randomizations.
Figure 1.
Study Design Flowchart
Neuroimaging Procedures
Image acquisition parameters are predefined to standardize the image acquisitions to the greatest possible extent across the centers which use a wide variety of imaging equipment makes and models. Each site is required to provide a test scan to the central imaging coordinating center for approval prior to site initiation and every 6–12 months during the enrollment phase.
MRI
All patients enrolled with MR imaging undergo MRI at 2 timepoints: prior to enrollment and at day 7. All MRIs are performed on MR scanners equipped with echo-planar imaging (EPI) capability to allow rapid acquisition of diffusion and perfusion scans. Patients with contraindications to MRI (metal implants such as pacemakers, claustrophobia, etc.) are not eligible for inclusion in the trial using MRI but could be eligible for inclusion using CT beginning July, 2009. The following sequences are obtained at both timepoints: diffusion-weighted imaging (DWI), Fluid-Attenuated Inversion Recovery (FLAIR), perfusion-weighted imaging (PWI), gradient recalled echo (GRE), intracranial time-of-flight magnetic resonance angiography (MRA), and contrast-enhanced neck MRA. A standard DWI sequence (b=0, 1000 s/mm2 applied in each of three principal gradient directions) is recommended. MRI perfusion measurements are made using dynamic T2*-weighted (gradient echo) EPI scanning. Early in the time series, a bolus (0.1 mmol/kg) of MRI contrast material is rapidly infused (5 ml/sec through an 18 or larger gauge angiocatheter) using a power injector.
CT
All patients enrolled with CT undergo multimodal CT imaging prior to enrollment and MR imaging at day 7 post-enrollment (unless MR is contraindicated in which case a follow-up MR RESCUE protocol CT can be performed). The following series are obtained at the pre-enrollment time-point: noncontrast CT, perfusion CT, and CT angiography of the head and neck vessels.
Immediately following acquisition of the baseline MRI or CT scan, the imaging data is transmitted to the dedicated MR RESCUE computer and the images are post-processed with the “RescueOnSite” program to determine the penumbral pattern. In brief, automated postprocessing 1) first classifies individual voxels within an at-risk region (defined as Tmax > 4 seconds within the affected hemisphere) as penumbral or likely to proceed to infarction, and then 2) classifies a patient as having an overall penumbral or non-penumbral pattern. The voxel based analyses incorporate information from the following sequences: MRI – apparent diffusion coefficient (ADC), Tmax (time to peak of the residue function), cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT); CT - Tmax (time to peak of the residue function), cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT). Separate publications will provide a description of the multivariate models, how they were derived for MRI and CT, and the detailed analyses performed in the “RescueOnSite” automated software. For both MRI and CT, a patient is classified as having a “penumbral pattern” if: 1) the irreversibly infarcted region identified by the voxel-based predictive model is ≤ 90 mL and 2) the ratio of the volume of penumbral tissue within the volume at-risk region is > 30%.
All images are subsequently securely transmitted to the centralized neuroimaging laboratory (initially located at the National Institutes of Health and transferred to Georgetown University in 2008) over the internet using a secure file transfer protocol (SFTP). At sites unable to execute SFTP, data is transferred to the neuroimaging coordinating center by hardcopy (e.g. compact disc).
Treatment or Intervention
Embolectomy Arm
Following the imaging study, if randomized to intervention, the patient is transported to the angiography suite. Diagnostic angiography is initially performed with catheterization of the carotid artery appropriate to the patient’s presenting symptoms. Once thrombus in the appropriate vessel is identified, the embolectomy procedure is initiated. From study launch in 2004 through 2009, the physician performing the embolectomy procedure used any approved Merci Retriever family device (Concentric Medical, Mountain View, California) as the initial endovascular therapy. After 2009, following FDA clearance of the Penumbra system (Penumbra Inc, Alameda, California), the physician performing the embolectomy procedure could use either a Merci Retriever or a Penumbra system device as the first device deployed. The choice of mechanical embolectomy approach is at the discretion of the treating physician based upon the location and extent of the lesion and vascular anatomy. If the initial device type (Merci or Penumbra) fails to recanalize the vessel, the other can be used as rescue treatment with the restriction that mechanical embolectomy procedures must be terminated by 9 hours past symptom onset. In addition, intra-arterial tissue plasminogen activator (tPA) up to a maximum of 14 milligrams is allowed as a rescue therapy initiated up to 6 hours past symptom onset in cases of treatment failure with mechanical embolectomy device, or to treat distal emboli not accessible to mechanical devices after successful proximal embolectomy. The maximum dose of 14 mg was selected as it is the average dose used in the Multi-Merci trial and was not associated with increased rates of symptomatic intracranial hemorrhage.(14) Intra-procedural systemic anticoagulation with heparin is left up to the discretion of the treating physician. If used, the maximum recommended dose is 2000 units of IV heparin bolus followed by 500 units of IV heparin every hour.
Interventionalists are directed to follow the Instructions For Use (IFU) for both the Penumbra system and Merci Retriever. The procedures for embolectomy using these devices have been previously described.(15, 16) Some procedural highlights are described below.
MERCI Retriever
The Concentric Balloon Guide Catheter (Concentric Medical, Mountain View, California) 8F or 9F is allowed. The microcatheters used for Merci retrieval are the Merci Microcatheter 14X or the Merci Microcatheter 18L (Concentric Medical, Mountain View, California). The choice of Merci retrieval device is left up to the discretion of the physician depending on target vessel size, anatomy and safety considerations. This includes any Food and Drug Administration (FDA) approved Merci device including the X5 and X6 (since the start of the trial), the L-series devices (L5 since 11/08/2005, and the L4, L6 and K-Mini since 6/27/2007) and the V-series devices (since 9/28/2008). The distal access catheters are also allowed to be used in the study (since 9/28/2008). As per the instructions for use of the Merci System, a total of six total passes are allowed.
Penumbra System
The Penumbra System, consisting of a reperfusion catheter and separator to access the occlusion, is available in different sizes according to the target vessel including the 032 or the 041 (since 8/13/2009) and the 054 (since 6/18/2010) reperfusion catheter/separator pair. The choice of reperfusion catheter is dependent on the target vessel size and is left up to the discretion of the treating physician. There is no restriction in time limit as to the use of the Penumbra System provided that the procedure does not continue beyond 9 hours post-ictus. Use of the system beyond a total of 120 minutes is discouraged.
Post procedure, a final arteriogram is required including an anterior-posterior (AP) and lateral view of the involved arterial system. Revascularization and reperfusion are interpreted centrally in the core lab by the interventional neuroradiologist principal investigator (RJ) employing the Thrombolysis In Myocardial Infarction (TIMI) scale as adapted for the cerebral circulation in the MERCI trial(12), the Arterial Occlusive Lesion (AOL) scale, and the Thrombolysis in Cerebral Infarction (TICI) scale.(17–19)
Concomitant Therapy and Standard Medical Management
Standardization of medical management is equivalent in both the embolectomy and control arms and conforms with the current American Heart Association / American Stroke Association guidelines.(20, 21) Sites are required to admit all study patients to a monitored or intensive care unit for at least 24 hours. Aggressive hypertensive-hypervolemic therapy is only allowed in the case of symptomatic blood pressure fluctuations or if blood pressure dropps below the normal range for the patient. All patients are placed on aspirin 325 mg each day for 7 days (clopidogrel can be used as adjunctive therapy if indicated for cardiac disease). After 7 days, the choice of antithrombotic therapy regimen is at the discretion of the treating physician. A follow-up imaging study is required in any patient with neurologic deterioration.
Statistics and Primary Outcome
The primary study hypothesis to be tested is that the presence of substantial ischemic penumbral tissue visualized on multimodal CT or MR imaging predicts patients most likely to respond to mechanical embolectomy for treatment of acute ischemic stroke due to a large vessel occlusion up to 8 hours from symptom onset. This hypothesis will be tested by analyzing whether pretreatment penumbral pattern has a significant interaction with treatment (embolectomy vs. conventional medical care) as a determinant of functional outcome based on the distribution of 90 day mRS scores across all 7 levels of the global disability scale (shift analysis). Two nested hypotheses will be tested if the primary study hypothesis is validated. These two nested hypotheses are: 1) patients with a penumbral pattern have improved functional outcome when treated with mechanical embolectomy vs. standard medical care, 2) patients without a penumbral pattern do not have improved functional outcomes when treated with mechanical embolectomy vs. standard medical management, and to determine in an exploratory manner if there is a signal of potential moderate efficacy or harm. An additional hypothesis will only be tested if the primary hypothesis of an interaction is negative: that patients treated with mechanical embolectomy have improved functional outcome vs. standard medical management (regardless of imaging pattern).
Secondary Outcomes
The following additional endpoints will be analyzed to compare a variety of clinical outcomes compared between and across all treated vs. untreated patients, all penumbral treated vs. untreated patients, and all non-penumbral treated vs. untreated patients.
-
Efficacy
mRS 0–2 vs. 3–6 at day 90
mRS 0–1 vs. 2–3 vs. 4–6 at day 90
Change in National Institutes of Health Stroke Scale (NIHSS) score from baseline to day 90
Global test statistic at day 90(22)
-
Safety
Symptomatic and asymptomatic intracranial hemorrhagic transformation at 24 hours and day 7
All serious adverse events
Day 90 mortality
-
Imaging
Percent of pretreatment penumbral tissue salvaged on day 7 imaging
Change in ischemic lesion volume through day 7
Mean day 7 final infarct volume
Recanalization (patency score) at day 7 based on MRA or CTA
-
Clinicoradiographic Correlations
Change in NIHSS score vs. percent penumbral tissue salvage
Change in NIHSS score vs. change in ischemic lesion volume
Predictors of symptomatic and asymptomatic hemorrhagic transformation
Baseline core lesion volume and baseline NIHSS score
Baseline perfusion lesion volume and baseline NIHSS score
Day 7 final infarct volume and day 7 NIHSS score
Additional post-hoc analyses will include comparison of outcomes in patients with recanalization vs. no recanalization in various imaging pattern and treatment groups as well as alternative or optimal definitions/models of core and penumbra.
Data Collection, Data Entry and Database
Clinical data collected on all patients includes patient demographics, previous medical history, vital signs, laboratory assessments, NIH Stroke Scale, Barthel Index, Glasgow Outcome Scale, Stroke Impact Scale and modified Rankin Scale scores at various timepoints. A schedule of events is provided in Table 2. Additional clinical information collected includes concomitant medications, complications of therapy, neurologic worsening, and all serious adverse events. Site personnel enter study data into a secure, interactive web-based database maintained at the UCLA coordinating center.
Table 2.
Schedule of Events
| Baseline | 24 Hrs (±6 hrs) | 72 Hours (±12 hrs) | Day 7 (±24 hrs) | Day 30 (±3 d) | Day 90 (± 7 d) | |
|---|---|---|---|---|---|---|
| Consent | ✔ | |||||
| History / Exam | ✔ | |||||
| Routine Labs | ✔ | |||||
| Vital Signs | ✔ | ✔ | ✔ | ✔ | ✔ | |
| EKG | ✔ | |||||
| IV tPA Form | ✔ | |||||
| MRI or CT | ✔ | ✔ | ||||
| NIHSS | ✔ | ✔ | ✔ | ✔ | ✔ | |
| GOS | ✔* | ✔ | ✔^ | ✔ | ||
| mRS | ✔* | ✔ | ✔ | ✔^ | ✔ | |
| Barthel Index | ✔* | ✔ | ✔^ | ✔ | ||
| SIS | ✔ | |||||
| Interval Events | ✔ | ✔ | ✔ | ✔ |
Scored for premordid state
Obtained through phone interview
Abbreviations: EKG=electrocardiogram, NIHSS = NIH Stroke Scale, GOS=Glasgow Outcome Scale, mRS = modified Rankin Scale, SIS=Stroke Impact Scale
Data Safety and Monitoring Board
The trial is monitored by an external, unblinded Safety Monitor and an external, NIH-appointed Data and Safety Monitoring Board (DSMB). All serious adverse events, whether or not considered to be related to study, are reported to the external, unblinded Medical Monitor. Study-wide SAEs are reported to site IRBs and federal regulatory agencies according to current guidelines. The DSMB formally reviews all SAEs to date at each annual DSMB meeting.
Sample Size
The sample size was calculated employing pilot data obtained from 23 patients undergoing endovascular embolectomy for treatment of acute ischemic stroke up to 8 hours from onset with pretreatment multimodal MRI imaging at UCLA and day 30 mRS scores available from 2001–2003. Based on this data, the following assumptions were made: 1) approximately 50% of patients will have a penumbral pattern, and 2) approximately 50% of patients undergoing embolectomy will have successful recanalization/reperfusion. Based on the pilot mRS outcome data and the above assumptions (Table 3), MR RESCUE has 74% power (α = 0.05) to detect an interaction between treatment assignment and penumbral imaging, and 79% power to detect a difference in outcome between the embolectomy arm and the control group independent of imaging pattern.
Table 3.
Modified Rankin Scale Means for Sample Size Calculation (n=23)
| Embolectomy | Control | All | |
|---|---|---|---|
| Penumbral | 3.05 | 4.60 | 3.825 |
| Non-penumbral | 4.45 | 4.50 | 4.475 |
| 3.75 | 4.55 | 4.15 |
Protocol Modifications
Major protocol modifications that occurred over time in the trial are listed in Table 4. Patients who failed treatment with IV tPA (demonstrated persistent neurologic deficit and persistent target occlusion on post-tPA infusion MRA or CTA) are included in the trial to increase enrollment without impacting the primary hypothesis. The Multi Merci results demonstrated no safety concerns with this approach.(14) Multimodal CT was added as a screening imaging modality to increase the number of sites and subjects eligible for enrollment following development of a voxel-based predictive model of ischemic core and penumbra as done with MRI. The MRI and CT models behave comparably. The Penumbra System was added as a device option for the mechanical embolectomy arm following its clearance by the FDA for removal of thrombi in patients with acute ischemic stroke and publication of results of the Penumbra System Pivotal Stroke Trial indicating safety and recanalization rates comparable to those of the Merci Retriever.(16) This decision was supported by a substantial number of participating interventionalists who indicated that they would like to have both the Merci Retriever and the Penumbra System as options for the embolectomy procedure.
Table 4.
Major Protocol Amendments
| Modification | Date Introduced |
|---|---|
| Patients treated with IV tPA without successful recanalization allowed as eligible for inclusion if persistent target occlusion visualized on post-tPA infusion MRA or CTA | January, 2008 |
| Multimodal CT allowed as screening imaging modality | July, 2009 |
| The Penumbra System introduced as option for embolectomy | October, 2009 |
Study Organization and Funding
The MR RESCUE trial is funded by the National Institute of Neurological Disorders and Stroke (NINDS grant # P50 NS044378) through the UCLA Specialized Program of Translational Research in Acute Stroke (SPOTRIAS) program project grant mechanism. Data management and biostatistical support are provided through the UCLA SPOTRIAS Biostatistics and Data Management Core. UCLA serves as the Clinical Coordinating Center and Georgetown University as the Neuroimaging Core Laboratory. A trial executive committee and an investigator-based steering committee have been established. A NINDS-appointed, independent DSMB oversees all trial activities. The trial is being performed under an FDA approved IDE (G050077).
SUMMARY
The MR RESCUE Trial is designed to test the penumbral imaging selection hypothesis for endovascular recanalization therapies for acute ischemic stroke. Specifically, the study is designed to demonstrate whether multimodal MRI and CT can identify patients who will benefit substantially from mechanical embolectomy up to 8 hours from symptom onset. If the primary aim is negative, a secondary aim will test the hypothesis that patients treated with mechanical embolectomy have improved functional outcome vs. standard medical management, regardless of penumbral imaging pattern. A total of 120 patients are being randomized to embolectomy vs. standard medical care, with randomization stratified by MRI pattern (penumbral vs. non-penumbral).
There are several unique aspects of the MR RESCUE trial: 1) it is currently the only randomized, controlled trial specifically designed to validate the imaging selection hypothesis for endovascular acute ischemic stroke therapies, 2) randomization is stratified by penumbral pattern ensuring adequate balance of groups, 3) automated image processing of penumbral pattern occurs on site in real time, 4) it will be the first randomized, controlled trial of mechanical embolectomy vs. standard medical management. Successful conduct of the trial will evaluate whether use of multimodal imaging is a rational and appropriate selection criterion for late recanalization therapies for acute ischemic stroke including mechanical embolectomy. A positive trial will suggest substantial clinical benefit from embolectomy therapy in the group of patients with a penumbral pattern.
Acknowledgments
This study is supported by a grant from the National Institutes of Neurological Disorders and Stroke (NINDS)/ National Institutes of Health (NIH), P50 NS044378. Concentric Medical, Inc. (Mountain View, CA) provided study catheters and devices from study start until August, 2007; thereafter, costs for all study catheters and devices have been covered by study funds or third party payors.
Footnotes
Conflicts of Interest and Disclosures:
Dr. Jahan has served on the speakers’ bureau for Concentric Medical, Inc. and serves as a consultant for ev3, Inc. The University of California, Regents receive funding for Dr. Saver’s services as a scientific consultant regarding trial design and conduct to ev3 and Grifols. Drs. Jahan, Alger, Starkman, Elashoff, Gornbein, Nenov, and Saver, and Ms. Guzy are employees of the University of California, which holds a patent on Merci Retriever devices for stroke.
References
- 1.Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997;28(4):866–72. doi: 10.1161/01.str.28.4.866. [DOI] [PubMed] [Google Scholar]
- 2.NINDS rt-PA Stroke Group. Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004 Mar 6;363(9411):768–74. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010 May 15;375(9727):1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004 Mar;61(3):346–50. doi: 10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 7.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011 Jul;42(7):1952–5. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51(2):418–26. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003 Nov;34(11):2729–35. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 10.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006 Nov;60(5):508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 11.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008 Apr;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 12.Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008 May;28(5):887–91. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatri P, Hill MD, Palesch YY, Spilker J, Jauch EC, Carrozzella JA, et al. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008 May;3(2):130–7. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008 Apr;39(4):1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 15.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005 Jul;36(7):1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 16.The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009 Aug;40(8):2761–8. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 17.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. New England Journal of Medicine. 1985;312(14):932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 18.Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008 Mar;29(3):582–7. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005 Nov;36(11):2400–3. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003 Apr;34(4):1056–83. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007 May;38(5):1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 22.Tilley BC, Marler J, Geller NL, Lu M, Legler J, Brott T, et al. Use of a global test for multiple outcomes in stroke trials with application to the National Institute of Neurological Disorders and Stroke t-PA Stroke Trial. Stroke. 1996;27(11):2136–42. doi: 10.1161/01.str.27.11.2136. [DOI] [PubMed] [Google Scholar]