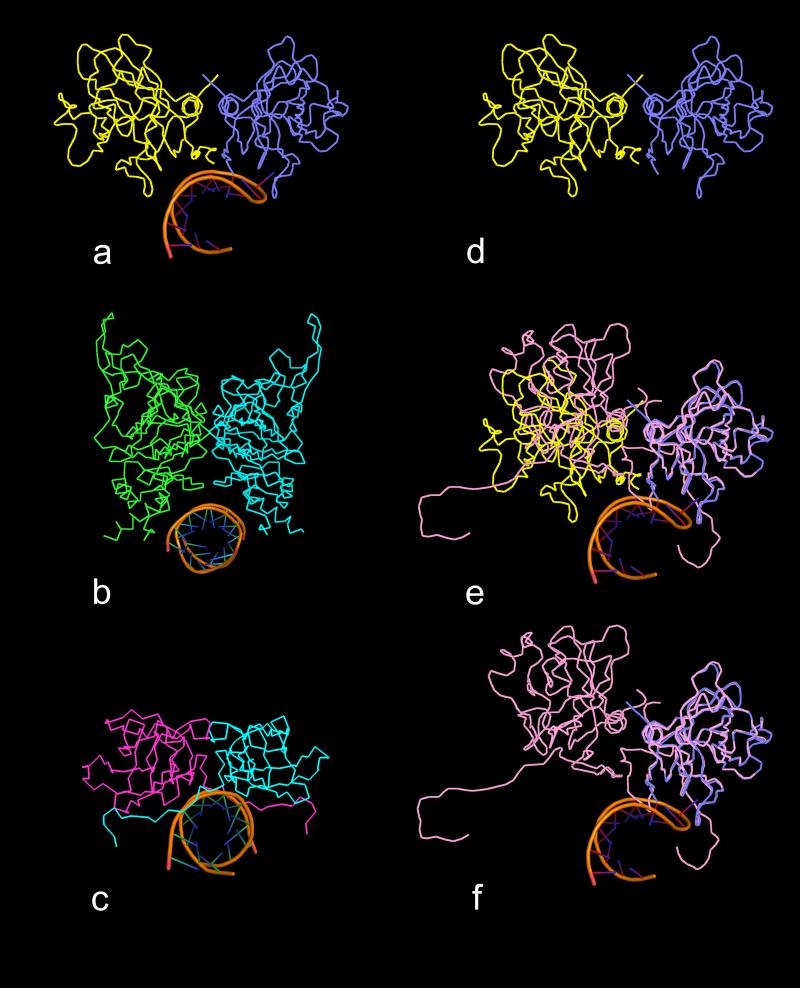
Fig. 12.
Comparison of binding sites of helical RNA structures with quasi twofold and exact twofold dimers. All views are down the helical axes of the RNA structures. In (a) is a ribbon diagram of the complex found in PMV in which the RNA is bound to the quasi twofold AB dimer (subunits A and B are blue and yellow, respectively). In (b) and (c) are the ribbon diagrams of the helical RNA structures that are bound to icosahedral twofold protein dimers in FHV and STMV, respectively. In each case, the protein dimer forms a clamp-like pocket around the RNA helix. In (d) is a ribbon diagram of the PMV AB dimer without RNA. In (e) the C1-C4 (see Fig. 4) exact twofold dimer (pink) has been superposed on the AB dimer using only Cα atoms of the A and C1 subunits. It is clear that the C4 and B subunits do not align well, illustrating the enormous difference between the quasi twofold and exact twofold dimers in PMV. In (f) the B subunit has been removed, revealing that the C-C dimer does not form a clamp-like structure that could wrap around an RNA helical structure, mainly due to the presence of the N-terminal arms of the two C subunits. These arms seem to simultaneously occupy the potential binding site and spread the dimer into a “flat” dimer versus the “bent” conformation of the AB dimer.