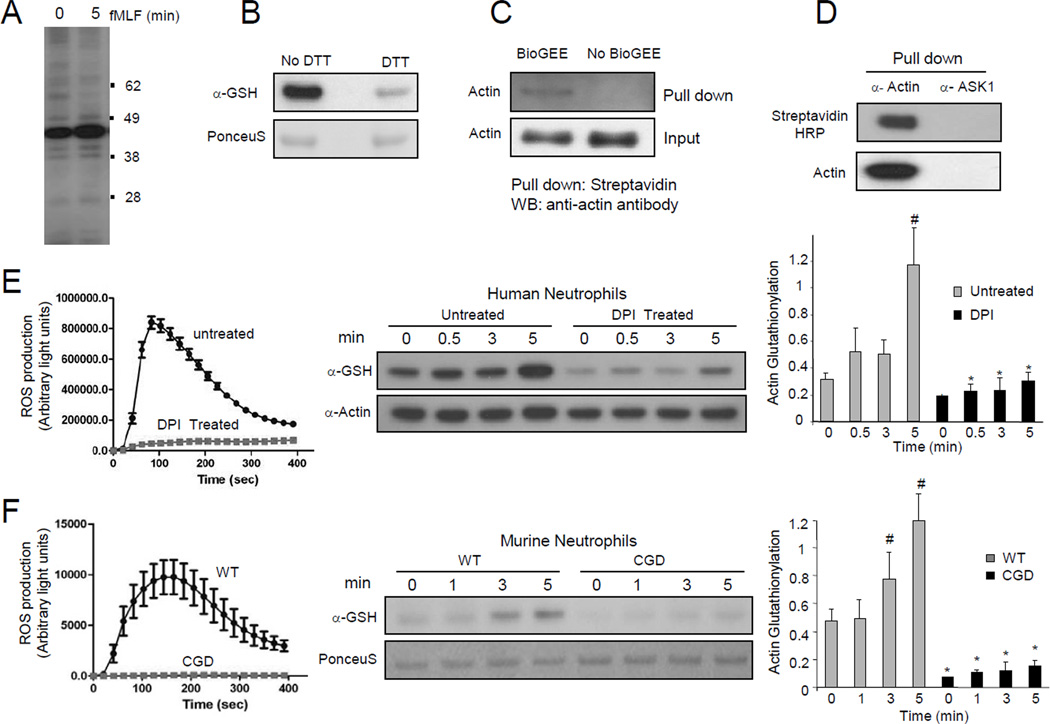
Figure 1. NADPH oxidase-mediated ROS production induced actin glutathionylation in chemoattractant-stimulated neutrophils.
(A) Protein glutathionylation in chemoattractant-stimulated human neutrophils. (B) Treatment with DTT leads to reduction of glutathione mixed disulfides. (C) Biotinylated glutathione (BioGEE)-modified proteins were pulled down from neutrophil lysates using Streptavidin agarose beads and probed with a β-actin antibody. (D) Actin was immunoprecipitated from BioGEE labeled neutrophil lysates (5 min after fMLF stimulation) and probed for Biotinylated-glutathione modification using Streptavidin-HRP. ASK1, a cytosolic protein, was used as a negative control. (E) Actin glutathionylation in fMLF-stimulated human neutrophils is dependent on NADPH oxidase activation. Human neutrophils pretreated with 50 µM DPI for 30 min were stimulated with 100 nM fMLF. Ratio of glutathionylated-actin to total actin is reported as actin-glutathionylation in the bar-graph (right). ROS production was evaluated by monitoring chemiluminescence (left). (F) Actin glutathionylation in fMLF-stimulated murine neutrophils stimulated with 1 µM fMLF. Data represents mean±SD from n=3 wells from one experiment representative of three. *, p < 0.001, versus WT. #, p < 0.001 versus time 0.