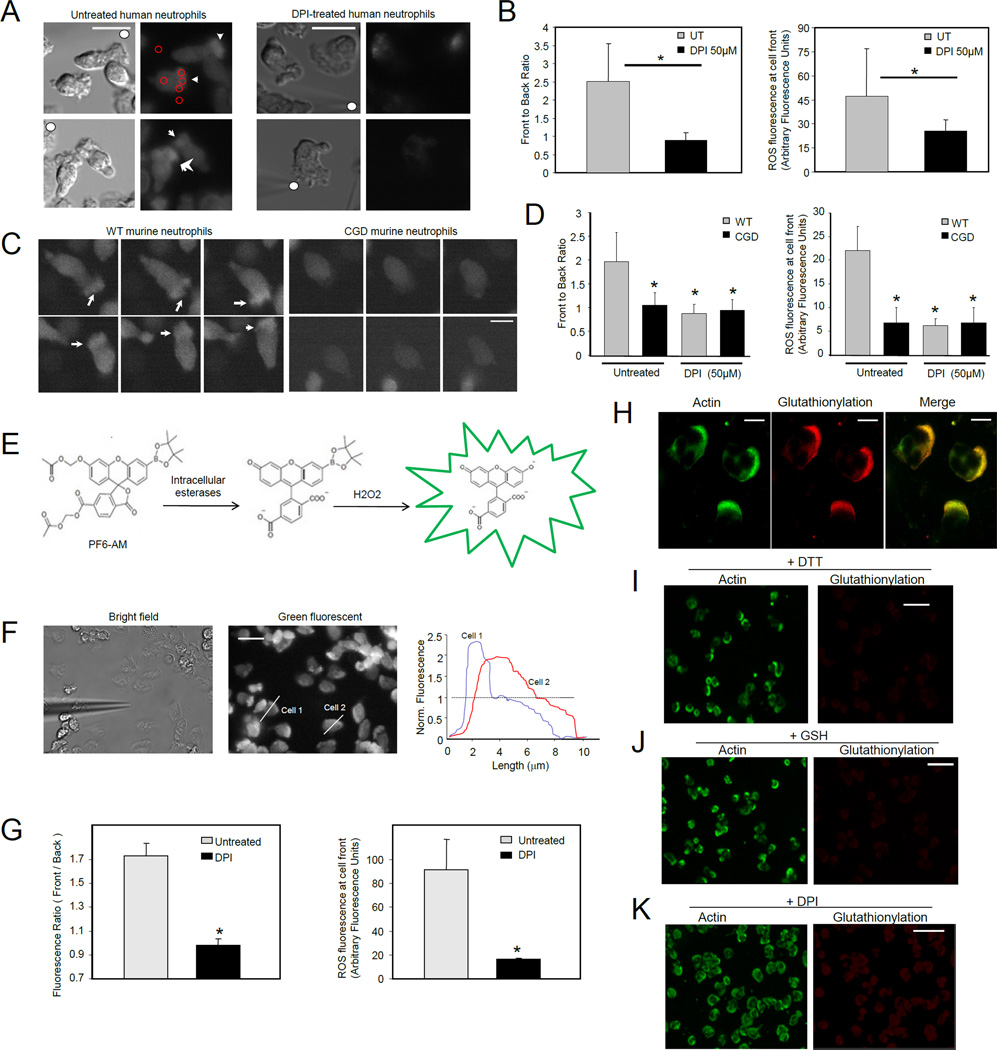
Figure 2. Both NADPH oxidase-mediated ROS production and protein glutathionylation occurred at pseudopodia of migrating human neutrophils, colocalizing with actin.
(A) Human neutrophils were labeled with ROS dye CM-H2DCFDA and exposed to a micropipette tip filled with 1 µM fMLF (denoted by solid white circle). Leading edges of migrating cells were marked by white arrows. Scale bars represent 10 µm. (B) Quantification of NADPH oxidase-dependent ROS localization at the front of migrating neutrophils. Four to five regions of interest (ROIs, red circles in Figure 2A) were randomly drawn at the front and back of the cell, as well as background regions of the image. Data are represented as mean±SD from n=12 cells. *, p < 0.001, versus untreated neutrophils. (C) Murine bone marrow neutrophils were labeled with 1 CM-H2DCFDA and exposed to a uniform bolus of chemoattractant (50 nM fMLF). Fluorescence images show randomly migrating neutrophils in three consecutive frames with 15 sec interval. Arrow heads point towards ROS localization at pseudopodia. Scale bars represent 5 µm. (D) Front to back ratio and ROS fluorescence intensity at the front of the untreated or DPI treated WT or CGD neutrophils were measured as described above. Data are represented as mean±SD from n=10 cells. *, p < 0.001, versus untreated WT neutrophils. (E) Mechanism of selective detection of intracellular H2O2 by PF6-AM. (F) Subcellular localization of H2O2 in chemotaxing neutrophils in a fMLF gradient. Human neutrophils were labeled with 1 µM of PF6-AM. Fluorescence intensity was measured by scanning a line (white line) through a cell with IPLab software. Shown are profiles of two representative cells. In this experiment, chemotactic gradient was generated with a micropipette filled with 10 µM fMLF. Scale bars represent 10 µm. (G) Front to back ratio and ROS fluorescence intensity at the front of the cell were calculated from images of untreated and 50 µM DPI-treated cells. Data are represented as mean±SD from n=15 cells. *, p<10−5 versus untreated neutrophils. (H) Distribution of glutathionylation and actin in fMLF-stimulated human neutrophils. Scale bars represent 5 µm. (I) Glutathionylation staining was conducted in the presence of 10 mM DTT. (J) Addition of excess amount of reduced GSH to primary antibody mix abrogated glutathionylation localization at the leading edge but did not affect detection of actin localization using the actin antibody. (K) Neutrophils were treated with 50 µM DPI before the fMLF stimulation. Glutathionylation staining was conducted as described in (H). The scale bars in I–K represent 20 µm.