Figure 2.
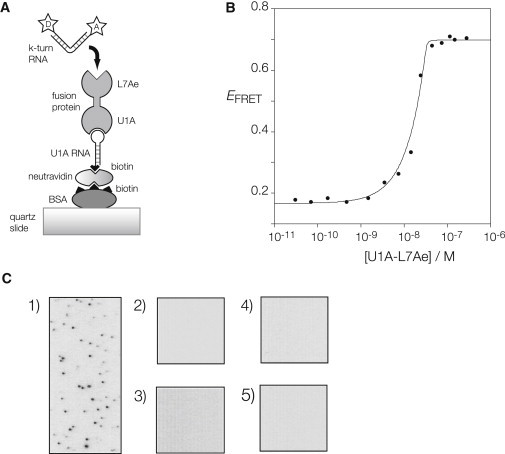
Binding of fluorescent k-turn-containing RNA to L7Ae protein immobilized on a microscope slide. (A) Schematic of the immobilization scheme. U1A-L7Ae fusion binds the loop of an RNA hairpin with a 5′-biotin that is bound to biotinylated BSA via neutravidin. Cy3-Cy5-labeled k-turn RNA flows into the cell, and binds to the L7Ae component of the fusion protein. (B) Induction of folding of Kt-7 upon binding of the U1A-L7Ae fusion protein. Protein-induced folding has been measured in bulk by means of FRET, using the same Cy3-Cy5-labeled RNA used in the single-molecule experiments. Folding kinks the RNA, leading to an increase in the efficiency of energy transfer between the 5′-terminally fluorophores. The plot shows FRET efficiency of Kt-7 as a function of protein concentration. The data have been fitted to a two-state model for L7Ae binding (line) using a stoichiometric binding model. The fitting indicates Kd < 10 pM, although such high affinity cannot be reliably estimated by this technique. The increase in FRET efficiency is ΔEFRET = 0.53, i.e., there is a large increase in FRET efficiency on binding the fusion protein. (C) When all the components are in place, individual bound RNA molecules can be visualized as single points of light (shown inverted as black) at Cy5 emission wavelength. (1) RNA bound to the complete set of components. No bound molecules are observed when the fluorescent RNA is added to (2) BSA only; (3) BSA + neutravidin only; (4) BSA, neutravidin, and U1A RNA only; or (5) BSA, neutravidin, U1A RNA, and U1A protein in place of the fusion.
