Figure 3.
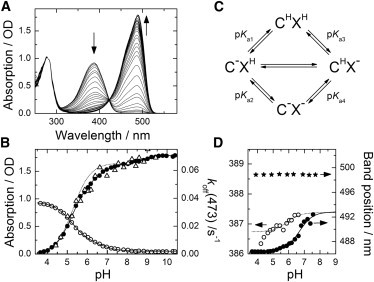
pH variation of the optical absorption spectra of mIrisGFP in the dark-adapted state (cis chromophore). (A) Absorption spectra in the pH range 3.6–10.4. Arrows point in the direction of increasing pH. (B) Peak absorption of the AC (open circles) and BC (solid circles) bands. Solid lines show the fit of the model depicted in panel c in the pH range 3.6–8. An additional protonation step described by the Henderson-Hasselbach equation was added to fit the BC band amplitudes at pH >8. Dotted line shows one-site protonation with pK = 5.3. Open triangles indicate pH dependence of the reaction rate coefficient, koff(473), for off-switching of the fluorescence with 473-nm light. (C) Four-state model describing the protonation equilibrium of the chromophore, C, interacting with a titratable amino acid, X. (D) pH dependence of the peak positions of the AC (open circles) and BC (solid circles) absorption bands of mIrisGFP, as well as the peak position of BC of the Glu212Gln mutant (stars).
