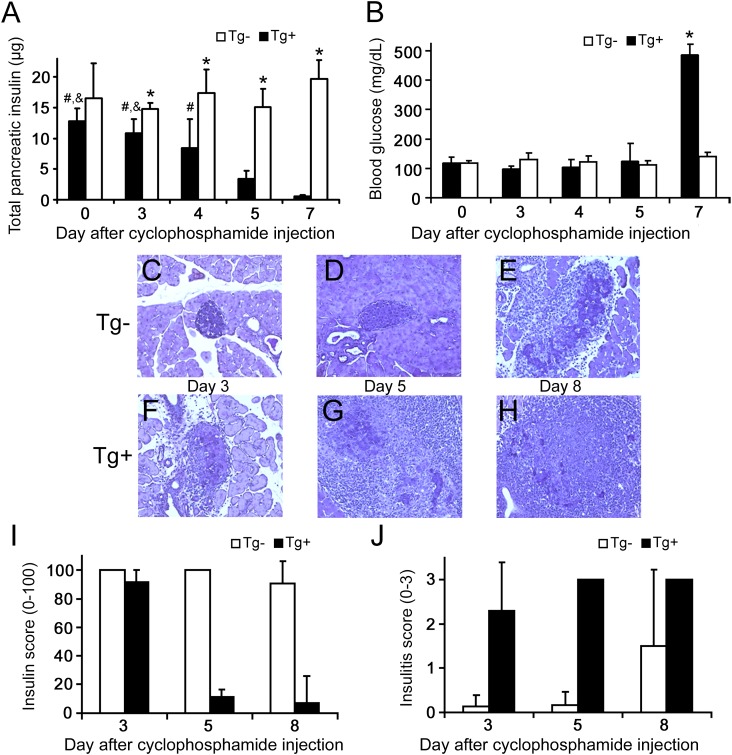
FIG. 1.
Characteristics of the cyclophosphamide-injected mouse model of type 1 diabetes. White bars, Tg− mice; black bars, Tg+ mice. All data are presented as mean ± SD. A: Total pancreatic insulin indicated the loss of BCM in Tg+ mice during the experimental time course (*P < 0.05 vs. same-day Tg+; &P < 0.01 vs. day 5 Tg+; #P < 0.01 vs. day 7 Tg+), whereas BCM remained constant after cyclophosphamide injection in Tg− mice. B: The decreased BCM in Tg+ mice was registered as an increase in blood glucose only after day 7, by which time loss of BCM had already occurred. C–H: Histological changes in cyclophosphamide-treated mice. Five-micron sections were stained with aldehyde fuchsin (deep violet) to visualize β-cell insulin granules; a hematoxylin counterstain (light blue) was used to identify nuclei and highlight areas of mononuclear infiltration. Representative sections are shown at ×200 magnification. C and D: Pancreatic infiltration was minimal before day 5 in nontransgenic mice, whereas invasive insulitis with some evidence of β-cell damage had developed by day 8 in some of these mice (E). In contrast, invasive insulitis was clearly evident by day 3 in Tg+ mice (F), with increasing evidence of destructive insulitis in all mice by day 5 (G). In the single mouse remaining nondiabetic by day 8, extensive islet damage was widespread, with only small numbers of residual β-cells retaining detectable insulin granules (H). No granulated β-cells were seen in sections from diabetic mice (not shown). I: Histological insulin score, indicative of BCM, decreased in Tg+ mice but remained relatively constant in Tg− mice. J: Histological insulitis score on a 0–3 scale.