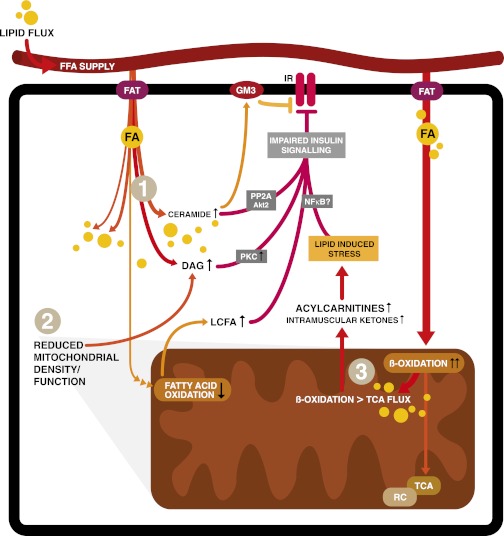
FIG. 2.
Mechanisms of lipid-induced insulin resistance. After transportation into the cell, FA can be stored, oxidized, or used as building blocks and signaling molecules (not all shown). Excess lipid supply and subsequent accumulation in insulin-sensitive tissues such as skeletal muscle is proposed to interfere with different insulin-responsive metabolic pathways via various mechanisms. Firstly (1), increased intracellular lipid content inhibits insulin signaling via lipid intermediates such as ceramides, diacylglycerol (DAG), or gangliosides (GM3) via effects on protein phosphatase A2 (PPA2) and protein kinase B (Akt), protein kinase C (PKC), or effects on the insulin receptor in the cell membrane (1,3,5–8,44). Effects of lipid intermediates on inhibitors of nuclear factor-κβ (NFκB) kinase subunit β and c-Jun N-terminal kinase 1 are not depicted. The second mechanism (2) is a decreased number of functional mitochondria resulting in lower FAO rates and increased accumulation of cytosolic lipid, again interfering with insulin sensitivity (2,9). Finally (3), metabolic overload of mitochondria leads to incomplete β-oxidation. In this figure, oxidation of FA outpaces the TCA and respiratory chain (RC), resulting in intramitochondrial accumulation of FAO intermediates like acylcarnitines. These subsequently impinge on insulin signaling (1,48,50–56). In this figure, only the direct effects of acylcarnitines on nuclear factor-κβ have been proposed (70).