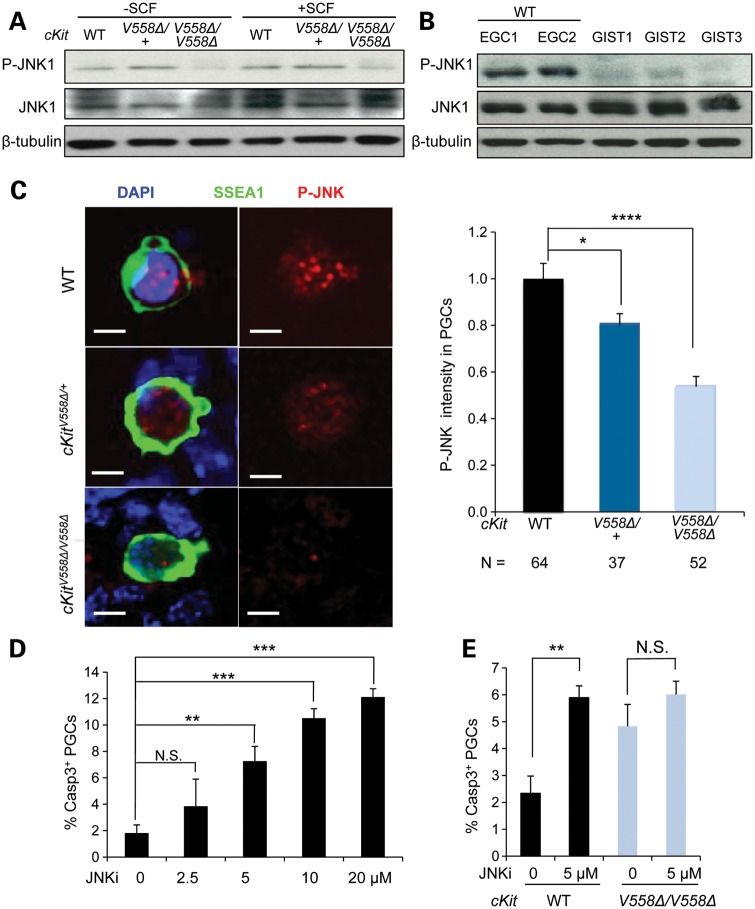
Figure 7.
Loss of JNK signaling compromises the survival of cKitV558Δ/V558Δ mutant PGCs. (A) EGCs sorted from STO cells were starved for 2 h and then treated with 50 ng/ml SCF for 10 min. Immunoblotting for P-JNK, total JNK and β-tubulin showed a decrease in JNK1 phosphorylation in cKitV558Δ/V558Δ EGCs. (B) Lysates of GISTs from three cKitV558Δ/+ mice were compared with cell lysates isolated from sorted EGCs (WT, two lines) by immunoblotting. P-JNK was not reliably detected in GIST lysates. (C) Gonadal cells from E11.5 WT, cKitV558Δ/+ or cKitV558Δ/V558Δ embryos were cultured for 20 h before P-JNK and SSEA1 co-immunostaining. P-JNK immunolocalized to the nuclei of PGCs and appeared reduced in cKitV558Δ/V558Δ PGCs, as shown by quantification of P-JNK intensity. Scale bars = 5 µm. (D) Gonadal cells from E11.5 WT embryos were cultured in DMEM with 50 ng/ml SCF at the indicated concentrations of a JNKi, SP600125, for 20 h. Apoptotic PGCs were detected by co-immunostaining with SSEA1 and caspase 3, with DMSO as a vehicle control. The JNKi increased PGC apoptosis in a dose-dependent manner. (E) PGCs cultured with gonadal somatic cells of E11.5 WT or cKitV558Δ/V558Δ embryos were treated with 5 µm JNKi for 20 h in the presence of 50 ng/ml SCF. The JNKi increased apoptosis of WT PGCs, but not cKitV558Δ/V558Δ PGCs. **P < 0.01, ***P < 0.001, ****P < 0.0001. Bars represent mean ± SEM.