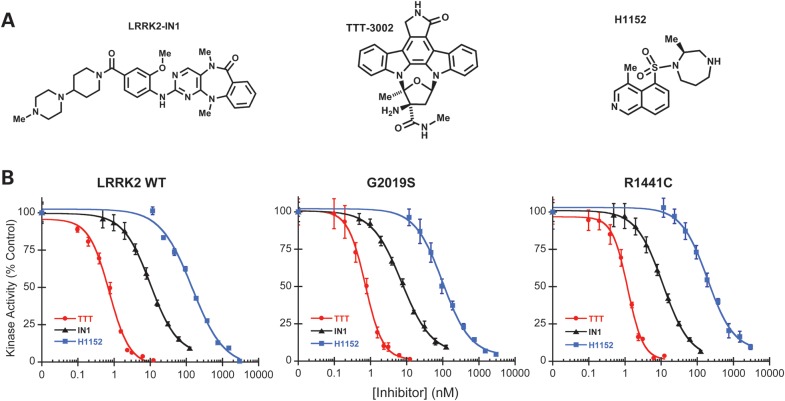
Figure 1.
Kinase inhibitory effects of LRRK2-IN1, TTT-3002 and H-1152 on recombinant LRRK2 and mutants in vitro. (A) Chemical structures of LRRK2-IN1, TTT-3002 and H-1152. (B) Inhibition curves of LRRK2-IN1, TTT-3002 and H-1152 for LRRK2 WT, G2019S and R1441C. Inhibitors were assayed in triplicate using 8 nm Invitrogen LRRK2, R1441C and G2019S separately in the presence of 100 μm ATP and 100 μm LRRKtide. The results are presented as percentage of kinase activity relative to the vehicle control from three independent experiments performed in triplicate. Data are expressed as mean ± SEM.