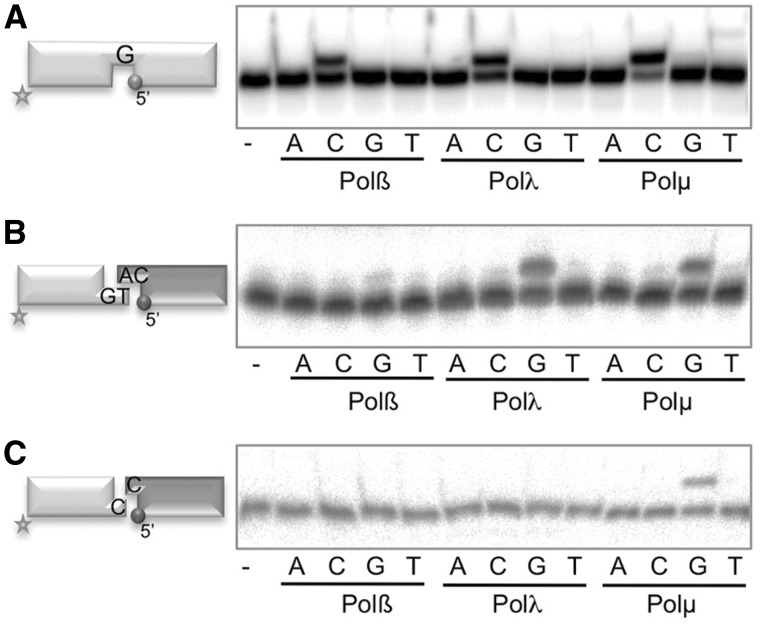
Figure 4.
Impact of Polµ DNA-binding properties on its enzymatic activity during NHEJ. (A) Gap-filling activity of Polβ, Polλ and Polµ (25 nM each) was assayed using a substrate formed by the hybridization of the oligonucleotides SP1C, T28 and D12. When indicated, 10 nM of each dNTP was added, in the presence of 2.5 mM MgCl2. (B) NHEJ assay of Polβ (600 nM), Polλ (600 nM) and Polµ (200 nM) was performed as described in ‘Materials and Methods’ section, using a set of compatible substrates: the labeled substrate was formed by hybridization of GT and NHEJ-D (shown in light gray) and the cold substrate by hybridization of CA and NHEJ-D (shown in dark gray). When indicated, dNTPs were added separately at 100 µM in the presence of 1 mM MnCl2 for Polβ and Polλ, and 2.5 mM MgCl2 for Polµ. After electrophoresis, the labeled fragments were detected by autoradiography. (C) NHEJ reaction performed as in (B), with a set of incompatible substrates in which both the labeled (light gray) and cold (dark gray) molecules were formed by hybridization of C- and D-NHEJ. When indicated, the substrates contain a 5′-P group at the downstream strand (dark gray spheres).