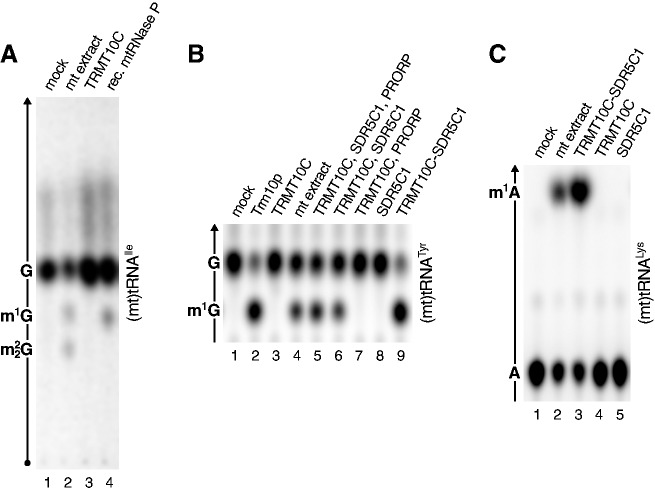
Figure 2.
A subcomplex of human mtRNase P has tRNA methyltransferase activity. (A) In vitro transcribed (mt)tRNAIle labelled by [α-32P]guanosine triphosphate was incubated with SAM and either HeLa cell mitochondrial extract (mt extract; lane 2), recombinant TRMT10C (lane 3) or mtRNase P reconstituted from its recombinant components (TRMT10C, SDR5C1, PRORP; lane 4). The tRNA hydrolysate was resolved by TLC; origin and direction of migration, and the positions of guanosine monophosphate (G) and its methylated derivatives (m1G and 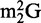 ) are indicated to the left. (B) (Mt)tRNATyr specifically labelled at the linkage between tRNA-position 8 and 9 only was incubated with HeLa cell mitochondrial extract (lane 4), with the indicated recombinant proteins (lanes 2, 3, 8), with combinations (mixtures) of those (lanes 5–7) or with recombinant TRMT10C and SDR5C1 purified as a complex (lane 9). The tRNA hydrolysate was resolved by TLC; direction of migration and the positions of G and m1G are indicated to the left; only the informative part of the TLC is shown. (C) (Mt)tRNALys labelled at the linkage between tRNA-position 8 and 9 was incubated with HeLa cell mitochondrial extract (lane 2), with the recombinant TRMT10C–SDR5C1 complex (lane 3) or with TRMT10C and SDR5C1 separately (lanes 4, 5). The tRNA hydrolysate was resolved by TLC; direction of migration and the positions of A and m1A are indicated to the left; only the informative part of the TLC is shown.
) are indicated to the left. (B) (Mt)tRNATyr specifically labelled at the linkage between tRNA-position 8 and 9 only was incubated with HeLa cell mitochondrial extract (lane 4), with the indicated recombinant proteins (lanes 2, 3, 8), with combinations (mixtures) of those (lanes 5–7) or with recombinant TRMT10C and SDR5C1 purified as a complex (lane 9). The tRNA hydrolysate was resolved by TLC; direction of migration and the positions of G and m1G are indicated to the left; only the informative part of the TLC is shown. (C) (Mt)tRNALys labelled at the linkage between tRNA-position 8 and 9 was incubated with HeLa cell mitochondrial extract (lane 2), with the recombinant TRMT10C–SDR5C1 complex (lane 3) or with TRMT10C and SDR5C1 separately (lanes 4, 5). The tRNA hydrolysate was resolved by TLC; direction of migration and the positions of A and m1A are indicated to the left; only the informative part of the TLC is shown.