Figure 4.
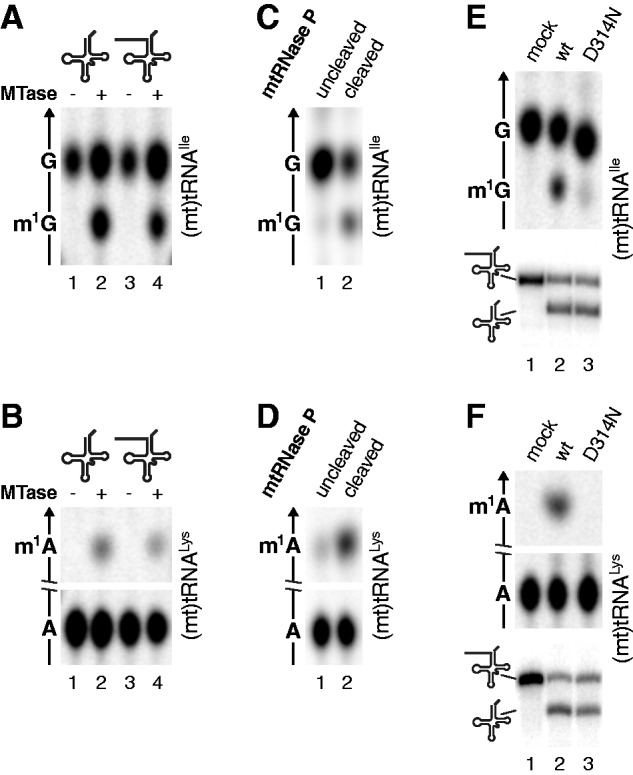
The methyltransferase activity of the TRMT10C–SDR5C1 complex and its contribution to mtRNase P activity are functionally independent. (A and B) A 5′ extension does not interfere with tRNA methylation. Position 9-labelled tRNAs with mature 5′ end (lane 2) or with 5′ extension (lane 4) were methylated with the TRMT10C–SDR5C1 methyltransferase (MTase) complex and the RNA hydrolysates resolved by TLC; (A) (mt)tRNAIle; (B) (mt)tRNALys. (C and D) Methylation and cleavage are not coupled. tRNA substrates with 5′ extension were partially (∼30%) cleaved with reconstituted mtRNase P (TRMT10C–SDR5C1, PRORP) in the presence of SAM. Uncleaved and cleaved tRNA were separated by PAGE, isolated and analysed for the presence of a methyl group at position 9 by hydrolysis and TLC; (C) (mt)tRNAIle; (D) (mt)tRNALys. (E and F) The functional integrity of the methyltransferase is not required to support cleavage by mtRNase P. tRNA substrates with 5′ extension were incubated with wild-type (wt) or mutant (D314N) TRMT10C-containing methyltransferase (TRMT10C–SDR5C1; upper panels) or reconstituted mtRNase P (TRMT10C–SDR5C1, PRORP; lower panels), and the reaction products separated by TLC (upper panels) or denaturing PAGE (lower panels; substrate and cleavage product indicated to the left); (E) (mt)tRNAIle; (F) (mt)tRNALys.
