Scheme 4.
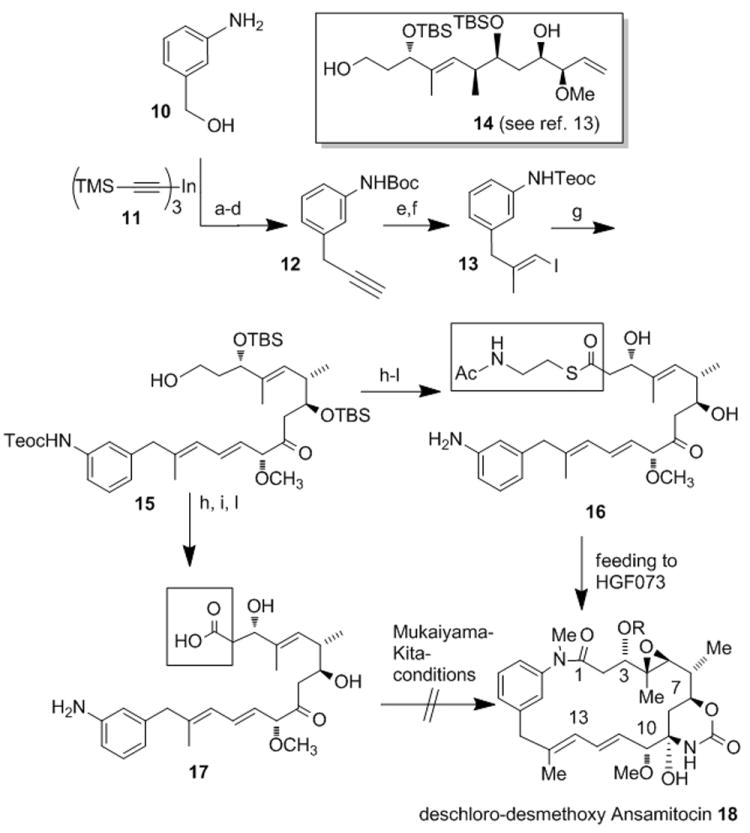
Total synthesis of SNAC ester 16 and seco acid 17[a] and feeding experiments with 16 towards deschloro-desmethoxy AP3 18.
[a] Reagents and conditions: (a) Boc2O, Et3N, dioxane/ H2O (2.5:1), rt, 12 h, (82 %); (b) PPh3, CBr4, CH2Cl2, rt, 1 h (83 %); (c) Pd(dppf)Cl2, THF, 65 °C, 4 h (98%); (d) TBAF*3H2O, THF, -20 °C, 1 h (88%); (e) i. AlMe3, Cp2ZrCl2, (CH2Cl)2, rt, 1 h, then addition of 11[13] at 0°C rt, 72 h, ii. I2, THF, -30 °C to 0 °C, 1.5 h (53%); (f) TeocCl, NaHCO3, CH2Cl2, rt, 10 min (91%); (g) 14, Pd(OAc)2, CsCO3, Bu4NBr, NEt3, DMF, rt, 2 h (68 %); (h) DMSO, (COCl)2, -60 °C, 1 h then Et3N, -40°C (90%); (i) NaClO2, NaH2PO4 H2O, t-BuOH, 2-methyl-2-butene, 0 °C to rt, 30 min (85%); (j) AcHN(CH2)2SH, DIC, DMAP, CH2Cl2, rt, 2 h (56%); (k) HF*pyr (70% HF) / THF, rt, 24 h (62 %); (l) ZnCl2, MeNO2, ultrasound, rt, 80 min (63 %; towards 17: 11%). (TMS= trimethylsilyl Boc= tert-butyloxycarbonyl; dppf= bis(diphenylphosphino) ferrocene, TBAF= tetra-n-butylammonium fluoride; DIC= diisopropyl carbodiimide, DMAP= 4-dimethylamino pyridine
