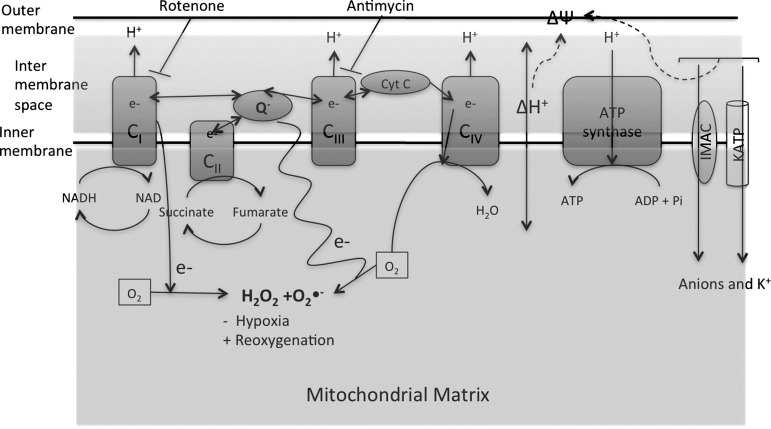
FIG. 3.
Synthesis of superoxide radical in the mitochondria. Electron transfer occurs between the Complex I through Complex IV of the respiratory chain. At the mitochondrial inner membrane, electrons from NADH (at Complex I) and succinate (at Complex II) pass to the coenzyme Q (ubiquinone [UQ]). UQ passes electrons to Complex III (cytochrome bc1 complex), which then passes the electrons to the cytochrome c (cyt c). Cyt c passes electrons to Complex IV (cyt c oxidase), which uses the electrons and hydrogen ions to reduce molecular oxygen to water. This enzymatic series produces a proton gradient across the mitochondrial membrane, producing a thermodynamic state that has the potential to do work. However, in this respiratory chain, a small percentage of electrons prematurely leak to O2 at complexes I, II, or III, resulting in the formation of the toxic O2•−. Reverse electron flow from Complex I also generated superoxide radicals. Inhibitors of Complex I and III that enhance the generation of ROS in cardiac mitochondria are Rotenone and Antimycin, respectively. Mitochondrial membrane potential (ΔΨm) is determined by the proton gradient across the membrane. Mitochondrial ion channels such as inner membrane anionic channel (IMAC) and ATP-sensitive K+ (KATP) channels cause ionic flux through the membrane and contribute toward mitochondrial membrane potential. The figure was created by adapting the information from (106, 195, 215), respectively.