Abstract
Biopharmaceuticals are drugs which are based on naturally occurring proteins (antibodies, receptors, cytokines, enzymes, toxins), nucleic acids (DNA, RNA) or attenuated microorganisms. Immunogenicity of these agents has been commonly described and refers to a specific antidrug antibody response. Such immunogenicity represents a major factor impairing the efficacy of biopharmaceuticals due to biopharmaceutical neutralization. Indeed, clinical experience has shown that induction of antidrug antibodies is associated with a loss of response to biopharmaceuticals and also with hypersensitivity reactions. The first disease-specific agent licensed to treat multiple sclerosis (MS) was interferon-β (IFNβ). In its various preparations, it remains the most commonly used first-line agent. The occurrence of antidrug antibodies has been extensively researched in MS, particularly in relation to IFNβ. However, much controversy remains regarding the significance of these antibodies and incorporation of testing into clinical practice. Between 2% and 45% of people treated with IFNβ will develop neutralizing antibodies, and this is dependent on the specific drug and dosing regimen. The aim of this review is to discuss the use of IFNβ in MS, the biological and clinical relevance of anti-IFNβ antibodies (binding and neutralizing antibodies), the incorporation of testing in clinical practice and ongoing research in the field.
Keywords: clinical testing, interferon-β, multiple sclerosis, neutralizing antibodies
Introduction
Multiple sclerosis (MS) is the most common inflammatory demyelinating disorder of the central nervous system. It is a chronic disabling disease largely affecting young people with onset typically between 20 and 40 years of age. MS has an incidence of approximately seven per 100,000 per year and a lifetime risk of 1:400. Worldwide it is estimated that MS affects 2.5 million people (approximately 100,000, i.e. ~1:600 people in the UK) and its economic impact is considerable [Compston and Coles, 2008]. It is considered the most common nontraumatic cause of chronic disability in young people [Sadovnick and Ebers, 1993]. Overall life expectancy is reduced by about 7 years and living with chronic disability is the major burden of the disease [Ragonese et al. 2008]. MS is characterized by episodes of neurological deficit followed by periods of remission and this relapsing and remitting nature is present in 85% of patients at onset. However, of these patients, around 90% develop a progressive form of disease over time: secondary progressive MS (SPMS) [Confavreux and Vukusic, 2008]. Up to 15% of people experience a progressive form of MS from onset, deemed primary progressive MS. In recent years there has been a rapid expansion in the number of disease-modifying agents licensed for MS, the majority of which have only been proven effective in the relapsing phase of the disease. Thus, it is essential to determine the clinical phenotype of the individual patient.
Pathologically MS is characterized by inflammation, demyelination, neuro-axonal loss and gliosis [Hohlfeld and Wekerle, 2004]. More recently the widespread involvement of grey matter, particularly early cortical lesions, has received much attention [Lucchinetti et al. 2011]. Current concepts of MS lesion formation are often based on findings derived from either experimental autoimmune encephalomyelitis, an animal model that resembles certain features of MS [Mix et al. 2008], biopsy or postmortem studies [Barnett and Prineas, 2004], which provide invaluable insights, though each representing only a limited aspect of the disease. Against this backdrop, MS has been described as a disease that is primarily mediated by autoreactive T cells (CD4+), which target specific epitopes in the central nervous system. Once activated, T cells produce an array of proinflammatory cytokines, which stimulate other T cells, B cells, natural killer cells, macrophages and microglia that in turn augment and perpetuate the inflammatory process which ultimately leads to neuronal loss and gliosis [Sospedra and Martin, 2005]. Potential targets for treatment include immune dysfunction, permeability of the blood brain barrier, components of the inflammatory cascade, putative autoantigens, demyelination, axonal loss (neuroprotection) and remyelination and regenerative processes (growth factors). Licensed therapies include the interferon-β (IFNβ) compounds IFNβ-1a (Avonex, Biogen-Idec, Massachusetts, USA; Rebif, Merck-Serono, Geneva, Switzerland) and IFNβ-1b (Betaseron/Betaferon, Bayer-Schering, Leverkusen, Germany; Extavia, Novartis, Basel, Switzerland), glatiramer acetate (GA) (Copaxone, TEVA, Petach Tikva, Israel), natalizumab (Tysabri, Biogen-Idec, Massachusetts, USA), fingolimod (Gilenya, Novartis, Basel, Switzerland) and mitoxantrone (Novantrone, Wyeth/Serono, Darmstadt, Germany). Notably there are several other agents in the pipeline which may be licensed in coming months. The IFNs and GA are first-line agents while natalizumab, fingolimod and mitoxantrone are reserved as second-line agents. Since the emergence of efficacious second-line agents, the onus on the clinician is to identify nonresponders and to offer an appropriate alternative treatment choice in a timely fashion.
Use of interferon-β in multiple sclerosis
IFNs are a family of proteins which stimulate inter- and intracellular responses to regulate viral infections, modulate the immune response and cell survival. Type 1 IFNs, namely IFNβ, were originally investigated as potential therapeutic agents in MS because of their antiviral activity [Borden et al. 2007]. Their effectiveness, however, is probably attributable to their numerous other immunomodulatory activities, including altering the T helper type 1 (Th1)/Th2 balance [Hussien et al. 2001], antagonizing proinflammatory cytokines (IFNγ, interleukin 12 and tumour necrosis factor-α), downregulating major histocompatibility class II expression, affecting antigen presentation [Yong et al. 1998; Yong 2002], antiproliferative effects on T-cell expansion, differentiation and increased T-cell apoptosis [Sharief et al. 2001; Yong 2002]. There is also evidence that type 1 IFNs inhibit transmigration of immune cells across the blood–brain barrier (BBB) [Leppert et al. 1996].
The first trial of IFNβ in MS was completed in 1993 and used IFNβ-1b. Since then, separate and comparative trials have been conducted for each new product. On average, all IFNβ products reduce the annualized relapse rate by approximately one-third [MS Study Group, 1993; Jacobs et al. 1996]. MRI indices show a 50–70% reduction in disease activity using conventional markers. Early use of IFNβ in subjects with a clinically isolated syndrome [Jacobs et al. 2000; Beck et al. 2002] has shown delayed time to first relapse and conversion to clinically definite MS. Current opinion favours starting treatment early in the course of the disease, as neurodegeneration (e.g. brain atrophy) can be detected from the very first manifestations of the disease, and at least a proportion of these degenerative changes may be secondary to inflammation [Frischer et al. 2009]. Hence, by starting treatment early this could delay the time to subsequent relapses and development of disability [Comi et al. 2001; Kappos et al. 2007; Clerico et al. 2008]. Efficacy regarding disability measures has been variable, with some trials showing an effect, others being inconclusive, and others that did not include disability as an outcome measure [Jacobs et al. 1996; PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group, 1998, 2001]. The IFNβs are generally well tolerated. Side effects include flu-like symptoms, injection site reactions, myalgia, abnormal liver function tests, anaemia, leucopenia and thrombocytopenia. Various strategies have been proposed to manage these side effects [Munschauer and Kinkel, 1997].
What are antidrug antibodies?
The breaking of immune tolerance to IFNβ, an alloimmune reaction, and the subsequent immune response is characterized by the production of antibodies. Binding antibodies (BAbs) are antibodies that bind to the drug but do not necessarily inhibit its biological action. BAbs may be detected within the first month of therapy. The rate at which they appear is dependent on the type of IFNβ used. For IFNβ-1b BAbs are detectable in most patients within 3 months [Ross et al. 2000], whereas for intramuscular IFNβ-1a treated patients, less than 10% were BAb positive at this time point. After 12 months, 97% of patients treated with IFNβ-1b, 58% of patients treated with subcutaneous IFNβ-1a and 33% of patients receiving intramuscular IFNβ-1a were BAbs positive. Although BAbs do not necessarily inhibit the biological action of IFNβ, as therapy is continued, maturation of the antibody body response may result in the production of high-affinity neutralizing antibodies (NAbs). NAbs are a subset of BAbs which prevent the binding of the IFNβ to its receptor on the surface of cells. When BAbs are detectable it is likely that NAbs are also present [Bendtzen, 2003], however their concentration and affinity generally increase as the response matures. Longitudinal studies of the development of BAbs and NAbs suggest that NAbs may develop as early as 4–6 months after the initiation of therapy [Pachner et al. 2005]. Generally, individuals who are likely to become NAb positive will do so within the first 2 years of treatment [Sorensen et al. 2005a]. The frequency of NAb positivity reported varies between products and assays used, however in general it is 2–6% for intramuscular IFNβ-1a, 15–30% for subcutaneous IFNβ-1a and 27–47% for subcutaneous IFNβ-1b [Farrell and Giovannoni, 2007]. Individuals who become NAb positive with low titres may revert to a NAb-negative status with time [Gneiss et al. 2004; Sorensen et al. 2005b]. This reversion has been more frequently reported in patients treated with subcutaneous IFNβ-1b rather than those receiving either IFNβ-1a compound.
Immunogenicity of interferon-β
The immunogenicity of IFNβ is dependent on a number of factors. These are product related (e.g. the presence of nonhuman sequences and aggregates), treatment related [e.g. quantity, frequency and mode of dosing (intravitreal, intravenous, subcutaneous, etc.) and half life] and patient related (e.g. genetics and concurrent illnesses). These factors have been covered in depth elsewhere [Singh, 2011; Farrell et al. 2012]. Studies have consistently shown IFNβ-1b to be significantly more immunogenic than IFNβ-1a. One factor thought to be of particular importance is the presence of aggregates, possibly arising from their very different methods of production. IFNβ-1b is produced as a recombinant protein in Escherichia coli, is unglycosylated and has a lower specific activity. The amino acid sequence also differs from that of endogenous human IFNβ in that a cysteine (position 17) has been substituted by serine, and methionine (position 1) has been removed. In contrast, IFNβ-1a is manufactured in Chinese hamster ovary cells, has an identical amino acid sequence to human IFNβ and has a glycosylation pattern [Conradt et al. 1987] that is similar to other mammalian proteins. Studies have shown that the greater immunogenicity and lower activity of IFNβ-1b is most likely a consequence of the lack of glycosylation [Runkel et al. 1998] and not a result of the changes in amino acid sequence, that is, changes that were added to increase stability. Glycosylation is thought to prevent a relatively hydrophobic region of IFNβ-1b from interacting with other protein molecules. The loss of glycosylation leads to the formation of aggregates, containing a mixture of disulphide-linked molecules, which are likely to be responsible for the observed increase in immunogenicity. These aggregates may be formed with other IFNβ-1b molecules or with the human serum albumin that is added to inhibit the formation of the IFNβ–IFNβ complexes. The manufacturing process is also likely to be an important factor in the formation of aggregates and their immunogenicity. Recent studies in transgenic mice immune tolerant for human IFNβ have shown that the presence of metal particles, of the type which may arise during manufacturing, greatly enhanced their immunogenicity [van Beers et al. 2012].
An increase in the understanding of the factors that lead to the enhanced immunogenicity of IFNβ has led to the reformulation of subcutaneous IFNβ-1a [Giovannoni et al. 2007]. Reformulation involved changes to the buffer system to increase IFNβ-1a stability and the removal of human serum albumin to prevent the formation of mixed albumin-IFNβ-1a aggregates. Clinical trials have shown the new liquid formulation to have a lower immunogenicity and an improved safety profile [Giovannoni et al. 2009]. However, patents on the early types of IFNβ have now expired and this has led to an expansion in the manufacture of biosimilars, of widely varying quality [Meager et al. 2011] and presumably immunogenicity.
The biological significance of neutralizing antibodies
IFNβ exerts its effects by initially binding to the cell surface type 1 IFN receptor complex. Formation of the IFNβ-IFN receptor complex leads to activation of the associated janus kinases (JAKs), which go on to phosphorylate the signal transducers and activators of transcription (STATs); this is known as the JAK-STAT signalling pathway [Platanias, 2005]. At a nuclear level, activation of transcription factors leads to the modulation of several hundred genes. Due to the large number of genes activated by IFNβ, the precise mechanism by which IFNβ acts is not understood. In order to identify patients who are responding to IFNβ it would be of great benefit to be able to use a biomarker that also confers a therapeutic effect. However, in the absence of such knowledge those which are reliably induced in response to IFNβ administration can be employed. Previous research has investigated the potential of a number of biomarkers including oligoadenylate synthetase [Pachner et al. 2003a], neopterin [Rudick et al. 1998], X-linked inhibitor of apoptosis factor 1 [Gilli et al. 2006], tumour necrosis factor apoptosis inducing ligand (TRAIL) [Wandinger et al. 2003; Gilli et al. 2006], β-2 microglobulin [Rudick et al. 1998; Francis et al. 2005], viperin [Pachner et al. 2009b], IFN-induced protein with tetratricopeptide repeats 1 [Pachner et al. 2009b] and myxovirus resistance protein A (MxA) [Deisenhammer et al. 2000; Pachner et al. 2003a]. The most specific and commonly used biomarker is MxA. Studies have consistently shown that treatment of patients with IFNβ leads to large increases in the concentrations of MxA mRNA, and in the presence of NAbs, MxA mRNA concentrations decrease in a titre-dependent fashion. Very high titres were associated with a complete loss of bioactivity [Deisenhammer et al. 1999; Bertolotto et al. 2003; Gilli et al. 2006; Hesse et al. 2009; Farrell et al. 2011]. The titre at which this loss of bioactivity occurred varied between studies ranging from any positive sample (NAb titre > 20 TRU/mL to > 600 TRU/mL) [Gilli et al. 2004; Sominanda et al. 2008] and is further discussed elsewhere [Polman et al. 2010]. This variation can be explained by the use of different types of NAb assays. Thus MxA induction has become the most commonly used biomarker to confirm IFNβ activity in vivo.
DNA microarrays have revolutionized the search for specific biomarkers for monitoring IFNβ activity in patients by enabling large-scale gene and genetic expression profiling. Recent studies have been reviewed in depth elsewhere [Comabella and Vandenbroeck, 2011]. Such studies have provided many possible targets, although further validation work is required.
The clinical significance of neutralizing antibodies
While the effect of NAbs on biomarkers of IFNβ activity can be easily demonstrated, the challenge has been to translate this into the clinical forum and treatment of the individual patient. The development of NAbs is considered by many to be a significant factor contributing to clinical treatment failure. In patients who remain NAb negative the reduction in relapse rate may be as high as 50% [MS Study Group, 1996; PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group, 2001]. It has been shown in numerous trials that patients who become antibody positive have higher relapse rates, lesion activity on magnetic resonance imaging (MRI), and in some studies a higher rate of disease progression. The clinical effect of NAbs has been shown to lag behind their appearance, and it is only after 12–24 months of treatment that the detrimental effect of NAbs becomes apparent [Clanet et al. 2002; Francis et al. 2005; Kappos et al. 2005]. The trials on which most of the evidence is based may be criticized for their small cohort size, duration of follow up, definition of NAb status and the assays used to test for NAbs. There is also a paucity of randomized, placebo-controlled studies which prospectively set out to answer this question (Table 1). Here we discuss the data from the pivotal IFNβ cohorts on which licensing was based. However, one must be aware of the pitfalls of deriving data from studies designed to show the clinical effect of IFNβ with regards to relapse rates and disease progression as opposed to investigating the clinical effect of NAbs. As NAb-positive subjects form around 30% of all treated patients, their absolute numbers are low and thus many studies are underpowered or are of short duration (as discussed by Polman and colleagues) [Polman et al. 2010].
Table 1.
Prospective randomized trials evaluating the effect of neutralizing antibodies on interferon-β efficacy in relapsing remitting multiple sclerosis.
| MSSG (IFNβ-1b, 250 µg) | PRISMS-4 (IFNβ-1a, 44 μg) | EDCT (IFNβ-1a, 30 and 60 µg) | |
|---|---|---|---|
| Annualized relapse rate | 13–36 months on study | 36–48 months on study | 12–48 months on study |
| NAb positive | 1.08 (n = 35) | 0.81 (n = 28) | 0.97 (n = 26) |
| NAb negative | 0.56 (n = 56) | 0.50 (n = 120) | 0.70 (n = 606) |
| (p < 0.05) | (p = 0.002) | (p = 0.04) | |
| MRI (new T2 lesions) | 24–36 months on study | 0–48 months on study | 12–36 months on study |
| NAb positive | 1.03 (n = 34) | 1.4 (n = 28) | 4.9 (n = 9) |
| NAb negative | 0.40 (n = 54) | 0.3 (n = 120) | 2.9 (n = 279) |
| (p = 0.067) | (p < 0.001) | (p = not significant) | |
| EDSS (sustained progression) | 0–36 months on study | Time to sustained progression prolonged | 0–48 months on study |
| NAb positive | –0.06 (n = 35) | EDSS not provided | 0.89 (n = 14) |
| NAb negative | +0.19 (n = 56) | 0.29 (n = 286) | |
| Not significant (p = 0.083) | (p = 0.01) | ||
| Conclusions | NAbs reduce clinical efficacy | NAbs reduce clinical efficacy | NAbs reduce clinical efficacy |
MSSG, MS Study Group [1996]; PRISMS-4, PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group [2001]; EDCT, [Kappos et al. 2005].
EDSS, Expanded Disability Status Scale; IFN, interferon; MRI, magnetic resonance imaging; NAb, neutralizing antibody.
The MS Study Group published several papers showing the efficacy of IFNβ-1b in reducing relapse rates and disease activity on MRI [MS Study Group, 1993; Paty and Li, 1993]. A further paper was published to discuss the impact of NAbs in more detail. In the treatment arm 35% of subjects became NAb positive by 18 months. Those who were NAb positive had higher annualized relapse rates from month 13 to month 36 than those who remained NAb negative (1.08 versus 0.56, p = 0.067). No difference in sustained progression [using the Expanded Disability Status Scale (EDSS)] was found. With regards to MRI parameters, subjects who were NAb positive had a higher accumulation of new lesions than those who were NAb negative during the third year (1.03 versus 0.4, p < 0.05). In this paper the authors concluded that NAbs did reduce the clinical efficacy of IFNβ [MS Study Group, 1996]. This original cohort was re-examined after 16 years to evaluate the relationship between short-term clinical outcomes (MRI activity and relapses) and disability [Goodin et al. 2011]. Of the original cohort, n = 372,260 subjects were identified and recruited into this follow-up phase more than 12 years after completion of the pivotal study. Of the 112 subjects who were not included, 80 had died and the authors comment that those not followed up had a ‘tendency to a more aggressive disease course’. In the interval between the pivotal study and the long-term follow-up treatment, exposure and disease monitoring was variable between subjects and thus a strategy of ‘high’ and ‘low’ exposure to disease-modifying drugs was employed to evaluate the effect of treatment on long-term outcomes. With regards to NAbs, seven subgroups were defined detailing NAb titre, persistence and reversion within the first 3 years of treatment. The authors did not find an association between NAb status in the randomized, controlled trial (RCT) with clinical outcome after 16 years but did describe better outcomes in subjects with higher total drug exposure. They thus concluded that NAbs have no bearing on long-term disability. However, in the RCT it was noted that NAb-positive subjects had higher relapse rates and more active MRIs than those who remained NAb negative. This highlights the ongoing debate of whether relapses are predictive of future disability. It would also be of interest to know about the treatment and NAb status of the 112 subjects lost to follow up.
The 4-year extension phase of the Prevention of Relapses and Disability by IFNβ1a Subcutaneously in MS (PRISMS) study showed a significant reduction in relapse rate and MRI activity in the patient group on high-dose subcutaneous IFNβ-1a. This effect was lost in those who developed NAbs [PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group, 2001]. The relapse rate was 0.5 for patients receiving 44 µg subcutaneous IFNβ-1a who were NAb negative and 0.81 in those who were NAb positive (an increase of 62%). There was also a significant difference in the number of T2 lesions on MRI. The median number of lesions was 0.3 in those who were NAb negative and 1.4 in the NAb-positive group. This annual increase in the T2 burden of disease was similar to that seen in the 2-year placebo arm of the study. Further analysis of these data evaluated 368 of the original patients with regards to NAbs [Francis et al. 2005]. In this analysis the majority of NAbs developed within the first 12 months of treatment. Thirty percent of the Rebif 22 µg group and 19% of the Rebif 44 µg group had a positive NAb result. At 12 months, relapse rates were the same at around 0.9, thereafter the NAb-negative cohort showed a steady linear decline in relapse rates over the next 36 months to 0.35. The NAb-positive group, however, had a fluctuating course, with relapse rates consistently higher than the NAb-negative group and similar to that for the placebo group. With regards to time to confirmed progression by one point on the EDSS scale, in the NAb-positive group 44% had confirmed progression compared with 40% of the NAb-negative cohort. Using interval positive analysis, however, a significant difference was found between the groups: NAb-positive/NAb-negative progression rate ratio 1.50, 95% confidence interval 1.03–2.17, p = 0.03. A significant difference was also seen in T2 MRI lesion load between the NAb-negative and NAb-positive groups. The median lesion load was 0.3 (mean 0.1) in the NAb-negative group and 1.4 (3.2) in the NAb-positive group. The median cumulative percentage change in T2 lesion burden from baseline to years 2 and 4 was –7.2% and –8.5% in the NAb-negative group receiving 44 µg three times weekly and 12.5% and 17.6% for the NAb-positive patients.
The European dose comparison trial evaluated the clinical significance of NAbs (intramuscular IFNβ-1a 30 µg versus 60 µg, once a week) [Kappos et al. 2005]. Patients were followed from baseline and at 3-monthly intervals for 48 months. Samples were evaluated for BAbs and NAbs (in BAb-positive patients). They found a higher proportion of NAb-positive patients in the 60 µg group. NAb-positive patients had a higher (39%) relapse rate from months 12 to 48. NAb-positive patients’ EDSS score progressed by a mean of 0.89 over 4 years compared with 0.29 in the NAb-negative group. With regards to MRI findings, both T1 and T2 lesion load were higher in the NAb-positive group from 24 months to the end of the study. This study is very important as it further clarifies the significance of NAbs.
The more recent BECOME study [Pachner et al. 2009a] compared the efficacy of IFNβ-1b with glatiramer and included analysis of the effect of NAbs on MRI outcomes over a 2-year period. The incidence of NAbs and effect of NAbs on bioactivity were consistent with previous studies. MRI outcomes in patients with NAbs at levels high enough to abolish bioactivity relative to patients without NAbs were analyzed. In those who had preserved bioactivity, the ratio of enhancing lesions per scan decreased from 7.6 in the pretreatment period to 2.6, equivalent to a 66% decrease in the post-treatment period. For the group that lost bioactivity, the reduction was only from 8.5 to 5.8, a 32% decrease. Thus, the loss of bioactivity due to high levels of NAbs resulted in reduced therapeutic efficacy of IFNβ-1b as manifested by diminished reductions in enhancing lesions on MRI. Relapse rates were recorded as a secondary outcome; however no difference was noted between the NAb-positive and NAb-negative cohorts. This is likely due to the study design, which was powered to detect change in MRI over a 2-year period. Experience from previous studies would suggest that to detect the clinical effect of NAbs, patients need to be followed for a longer period (e.g. PRISMS-4 and European dose comparison trial).
The finding that NAb-positive subjects tend to have fewer relapses in the first 6 months has been described in an independent Danish longitudinal study [Sorensen et al. 2007]. The results from the Danish study showed that NAb-positive patients had a significantly lower relapse rate in the first 6 months than in the NAb-negative cohort but that after 6 months this reversed and they experienced higher numbers of relapses. This finding is interesting as it implies that those who develop NAbs may do better in the first year of treatment than those who are NAb negative. This would suggest a difference in the immune response of subjects who develop NAbs to treatment. The other hypothesis is that BAbs, which precede the development of NAbs, may extend the half life of circulating IFNβ, thereby increasing its bioavailability [Sorensen et al. 2007]. This may also explain why studies of short duration may note increased MRI activity in people with NAbs without increased relapse rates.
It is also important to highlight studies by other groups reporting no difference in clinical parameters with regards to NAb status. In a study published by Goodin and colleagues, serum samples were tested by the pharmaceutical company at the physicians’ discretion rather than testing all subjects treated with the drug. Indications for testing were given with the request and these were used as clinical data. No relationship was noted between treatment failure and clinical disease progression and the presence of NAbs. However, one must question the accuracy of clinical data when not collected prospectively within a clinical trial [Goodin et al. 2007b].
More recently the BENEFIT [Kappos et al. 2007] study also reported no difference in the time to clinically definite MS (CDMS), or higher annualized relapse rate, in those who were NAb positive. However, of the original 368 subjects recruited only 112 developed CDMS during the study period, with 69% of the original placebo arm completing the study compared with 80% of the initial treatment arm. This may account for very low relapse rates in both groups and thus no detectable difference between the study arms.
One of the largest and most recent studies addressing the question of NAbs was the BEYOND study [Goodin et al. 2012]. This was a large multicentre trial evaluating the efficacy tolerability and safety of high-dose subcutaneous IFNβ-1b 500 µg three times a week versus standard dose subcutaneous IFNβ-1b 250 µg three times a week versus subcutaneous GA 20 mg daily. Patients were followed for a minimum of 2 years and up to 3.5 years (mean 2.3 years). Outcomes measured included clinical data regarding relapses, EDSS, NAb status (MxA induction assay) and MRI (T2 lesion load and volumes). Subjects were subdivided into always NAb negative (NAb titre < 20 NU) and eventually positive (last sample NAb titre >20 NU). During the course of the study, up to 40% of each treatment dose IFNβ cohort tested positive for NAbs but around 35% had reverted to NAb-negative status by the end of the study period (all originally having low positive titres). In general, no increased risk of relapse was found in the eventually NAb-positive group compared with the always negative group. There was a trend to shorter time to next relapse in NAb-positive subjects treated with the 250 μg dose and this was significantly different from the NAb-positive (high) subjects treated with 500 μg IFNβ-1b. The authors do not, however, describe the duration of NAb positivity in those deemed eventually NAb positive, which may be important as the clinical effect of NAbs and the effect on MRI parameters lags behind their emergence, as previously discussed.
In this paper, however, the effect on MRI markers of disease activity, namely new T2 lesions and total T2 lesion volumes, is marked with a dose-dependent effect of NAbs. Based on the findings of the important longitudinal MRI studies [Fisniku et al. 2008] it is known that the accumulation of new T2 lesions and increased T2 lesion volumes early in the disease course are predictors of poorer clinical outcomes, SPMS and disability. Thus if one believes MRI to be a reliable biomarker of MS disease activity, the authors of the BEYOND study must conclude that NAbs have a detrimental effect on disease course compared with those remaining NAb negative. In view of the relatively short study duration (mean follow up 2.3 years), perhaps this contributes to the apparently paradoxical findings reported.
These conflicting findings highlight how imperative prospective, long-term studies are in the assessment of the clinical impact of NAbs, as their effect appears to become more apparent with time and lags well behind their initial appearance.
Testing binding antibodies and neutralizing antibodies in the clinical setting
Binding antibody testing
A number of methods have been developed for the measurement of BAbs and these include Western blotting, radioimmunoassay and the most commonly used method, enzyme-linked immunosorbent assay (ELISA). ELISA-based methods have been widely used to provide a quantitative measure of BAbs. The most commonly used method is the capture ELISA [Pachner et al. 2003b], which has been found to give results that correlate more closely with those from NAb assays. In this method IFNβ is bound to a microtitre plate using an anti-IFNβ monoclonal or polyclonal antibody. Patient serum is incubated with the bound IFNβ and bound human antibodies detected using an enzyme-labelled antihuman antibody. A standard curve is constructed using a known positive BAb sample and sample values related to this to generate a titre.
Measurement of BAbs by Western blotting [Gneiss et al. 2008] involves the separation of the IFNβ by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer onto a nitrocellulose membrane. Membrane strips are incubated with diluted patient serum, a positive control (mouse anti-IFNβ) and a negative control. Bound human antibodies are detected by using a labelled antihuman antibody. Samples are considered positive when the band for the positive control is more intense than that for the negative control. The disadvantages of the assay are that it is not quantitative and results in relatively high numbers of false negatives. Radioimmunoassays [Lawrence et al. 2003] provide a quantitative measure of BAbs and involve the incubation of serum samples with 125I-IFNβ followed by separation of the bound and unbound tracer. The activity of the bound tracer is measured and the amount of 125I-IFNβ present quantified. The method is technically difficult to perform and has not been widely adopted.
Neutralizing antibody testing
Quantifying NAbs requires the measurement of the loss of the biological activity of IFNβ. A number of methods have been developed for the measurement of NAbs and all are based on measuring the in vitro responses of IFNβ-sensitive human cell lines to the application of IFNβ. Binding of IFNβ to the IFN receptor complex on the cells leads to a change in the expression levels of many genes, including those which have antiviral, antiproliferative and immunological properties. In the presence of NAbs these changes are inhibited. Four assays are described below which make use of these properties of IFNβ or of the IFNβ-induced expression of specific genes.
The cytopathic effect assay
The cytopathic effect (CPE) assay is recommended by the World Health Organization (WHO) [WHO, 1985] for the measurement of NAbs and is considered the gold standard method with which all other methods should be compared. In the assay, a virus-susceptible cell line is treated with patient serum and IFNβ prior to treatment with the virus. Cells that are stimulated by the IFNβ to produce antiviral factors remain viable and are quantified. NAb titres are calculated using the Kawade method [Grossberg et al. 2001a, 2001b]. Extensive work has been conducted to standardize the assay. However, it is far from ideal as it takes days to perform, is prone to variation and is subject to interference due to the presence of other antiviral factors within the serum.
Assays based on the interferon-β-stimulated production of myxovirus resistance protein A
Treatment of IFNβ-sensitive human cell lines leads to the production, in a dose-dependent manner, of the biomarker MxA. In assays using the biomarker MxA, cells are treated with IFNβ in the presence of patient serum, resulting in the production of MxA mRNA and MxA protein. MxA mRNA may be quantified by reverse transcription followed by real-time polymerase chain reaction (PCR) [Bertolotto et al. 2007]. The assay is much faster than the CPE assay; it is very reliable and reproducible but is relatively costly. MxA protein concentrations may easily be measured by ELISA, however a number of other methods have also been used, including an immunochemiluminescent method [Kob et al. 2003] and fluorescence-activated cell sorting [Vallittu et al. 2002]. The ELISA method is simple and faster than the CPE assay. The disadvantages are that the results are more variable than the real-time PCR-based method and the antibodies are not commercially available.
The reporter gene assay
In the quest to develop simple cost-efficient assays to test for NAbs, a reporter gene assay has been developed [Farrell et al. 2008; Lam et al. 2008]. The assay uses a stably transfected human fibrosarcoma HT1080 cell line (clone HL116), which contains a luciferase gene under the control of the IFN-stimulated response element. Activation of the JAK/STAT pathway by IFNβ leads to the production of luciferase. The luciferase may be quantified by the addition of its substrate luciferin and measurement of the intensity of the chemiluminescence produced. Patient serum is preincubated with IFNβ and added to the cells and further incubated. Luciferin substrate is added and the chemiluminescence measured. In the presence of NAbs the expression of luciferase is reduced. Titres are calculated using the Kawade method. The method is relatively simple, repeatable and can be completed in a single day [Farrell et al. 2011].
The in vivo induction of myxovirus resistance protein A
Following treatment of patients with IFNβ, an increase in the biomarker MxA occurs and is maximal at about 12 h post injection [Bertolotto et al. 2003]. If the patient has NAbs to IFNβ the amount of MxA produced is reduced or, in the case of particularly high NAb titres, abolished [Bertolotto et al. 2003; Hesse et al. 2009; Malucchi et al. 2011]. To deliver such testing in the clinical arena, blood samples must be collected into special tubes designed to lyse the cells and stabilize the mRNA. The mRNA is extracted, converted to cDNA by reverse transcription and analyzed by real-time PCR.
Incorporation of testing into routine clinical practice
There has been much controversy with respect to the significance of these antibodies in patients with MS who have been treated with IFNβ and how to manage them [Hartung and Munschauer, 2004; Farrell and Giovannoni, 2007]. Conflicting guidelines have been issued by several consensus groups [Sorensen et al. 2005a; Goodin et al. 2007a; Polman et al. 2010]. What has been agreed is the biological evidence that NAbs abrogate IFNβ biological efficacy. However, incongruous clinical data, as previously discussed, have resulted in ambivalence in clinical practice. In clinical practice one needs to acknowledge the highly variable responses which are seen between individuals. The inherent difficulty in monitoring response is that in the natural history of MS, relapses decline over time and progression develops. MRI is the most sensitive biomarker of subclinical MS disease activity, and the accumulation of new lesions predicts future disability [Fisniku et al. 2008]. However, there is a lack of correlation between lesions and relapses at an individual level, suggesting a need for other biomarkers [Sormani et al. 2009]. In subjects treated with IFNβ, NAbs are a predictor of increased disease activity as defined by new lesions on MRI and occurrence of relapses. It is also apparent that in the majority of RCTs conducted for 36–48 months an effect of NAbs on clinical outcome is reported and the results are conflicting in those of shorter duration.
The European Federation of Neurological Societies [Sorensen et al. 2005a] recommended that patients treated with IFNβ be tested for the presence of NAbs after 12 and 24 months of therapy (level A recommendation). For those who remain NAb negative at 18–24 months, further testing is not routinely required (level B recommendation). Class 1 evidence shows the presence of NAbs significantly reduces the effect of IFNβ on relapse rate, active lesions and burden of disease as seen with MRI. In patients who are NAb positive, measurements should be repeated at intervals of 3–6 months and therapeutic options should be re-evaluated (level A recommendation). Therapy with IFNβ should be discontinued in patients with high titres of NAbs at repeated measurements at intervals of 3–6 months (level A recommendation). In contrast, the 2007 recommendations by the American Academy of Neurology [Goodin et al. 2007a] concluded that there is probably a reduction in efficacy of treatment because of NAbs, and there is likely to be greater antibody production in response to IFNβ-1b than to IFNβ-1a, but they were unable to make definite recommendations for changing therapy.
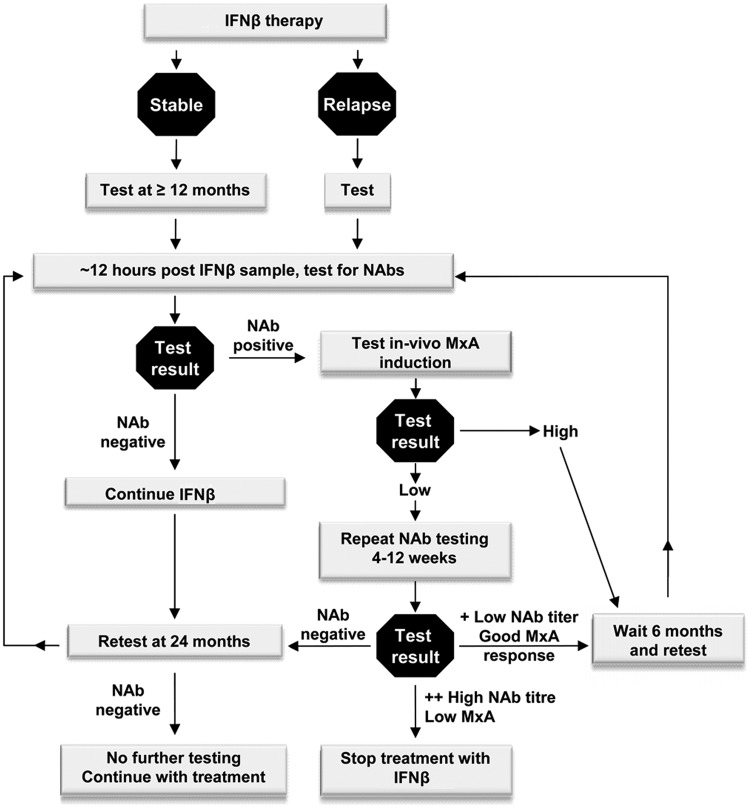
NABINMS (Neutralizing Antibodies to IFNβ in MS) was a European-wide network established in 2006 (until 2009) and funded by the European Union to provide standardized testing of NAbs and to define clinically relevant titres. The fundamental aim of this effort was to increase efficiency in the use of IFNβ in MS by avoiding the use of this treatment in patients in whom the drug is not effective. To achieve this, several goals were identified: validation and standardization of existing assays to test NAbs; development of new assays; evaluation of clinical effects in large cohorts of patients; evaluation of biomarkers of IFNβ bioactivity; and study of immunogenicity. Much of the work and opinion of this consortium are highlighted in the consensus paper of Polman and colleagues [Polman et al. 2010]. In this paper the authors also discuss the pitfalls of using data from studies designed to show the clinical effect of IFNβ with regards to relapse rates and disease progression and that as NAb-positive subjects form around 30% of all treated patients their absolute numbers are low. They concluded that there was evidence that NAbs have an impact on the biological efficacy of IFNβ and that all subjects treated for over 1 year should have NAbs tested. In those doing well on treatment who have persistent high-titre NAbs, a switch to an alternative treatment should be considered. In those with intermediate disease activity, continuation of therapy could be considered in those who are NAb negative but those who are NAb positive should switch to a non-IFNβ treatment. Patients whose condition has failed to respond to IFNβ treatment irrespective of NAb status should switch to an alternative treatment. We have included aspects of these recommendations in the algorithm detailed in Figure 1.
Figure 1.
Suggested algorithm incorporating neutralizing antibody testing in clinical decision-making process.
IFN, interferon; MxA, myxovirus resistance protein A; NAb, neutralizing antibody.
The knowledge that biopharmaceuticals lead to the development of antidrug antibodies has led to a second European collaborative initiative. Anti-Biopharmaceutical Immunization: Prediction and Analysis of Clinical Relevance to Minimize the Risk (ABIRISK, http://www.abirisk.eu) is an Innovative Medicines Initiative funded project set up to investigate the diverse factors that appear to be involved in the immunogenicity of biopharmaceuticals. The project involves collaboration between 35 partners (with varied disease interests) from 13 countries, and consists of 24 academic institutions, nine members of the European Federation of Pharmaceutical Industries and Associations (EFPIA) and two small and medium-sized enterprises. The objective of the project is to enable the development of tools that will be better at predicting immunogenicity during drug development and will ultimately lead to fewer immunogenic drugs.
Conclusion
The natural history of MS is highly variable and the modest effects of IFNβ in stabilizing disease are reflected in the 30% reduction in relapse rate reported in the pivotal studies. The body of evidence, measuring biological activity of IFNβ in the presence of NAbs, shows that NAbs confer a significant reduction in biological activity of the drug. Studies showing loss of clinical effect, as determined by MRI activity, are also convincing but data regarding relapse rates and effect on disease progression have been less so, with some reports which did not detect any clinical impact of NAbs. The problem of antidrug antibodies has been accepted with regards to many other biopharmaceuticals (e.g. natalizumab, insulin, factor VIII), and in an era of rapid change in MS therapeutics, identification of nonresponders to disease-modifying drugs and provision of an individualized treatment strategy are essential. The clinical effect of NAbs has been shown to lag behind their initial appearance and thus may be interpreted as heralding future treatment failure. The weight of evidence supports the opinion that high-titre NAbs reduce the bioefficacy of NAbs (in vivo biomarker induction, MRI parameters) and that in longer-term studies increased relapse rates are frequently reported. As screening for NAbs is now readily available in most MS centres and is a useful adjunct to the clinical decision-making process, consensus opinion recommends that NAbs should be evaluated within 12 months of treatment commencement. In those who are NAb negative further testing is only required with clinical indication or 12 months later [Polman et al. 2010]. In those who are NAb positive in vivo MxA induction is useful to further assess evidence of IFNβ bioactivity. In those who have persistent high positive NAbs and loss of MxA induction, the weight of biological data suggests that benefit from ongoing IFNβ administration is unlikely and thus, in view of the expanding treatment options available to patients with MS, an alternative treatment should be considered and guided by the clinical status of the individual. Similar personalized approaches should also be developed in non-IFNβ immunogenic treatments within the MS field and beyond.
Footnotes
Funding: This work was supported by the EU Framework 6 program ‘NABINMS’ (grant number LSHB-CT-2005-018926); the Barts and The London Charity (grant number 699/984) and by the Innovative Medicines Initiative Joint Undertaking (grant number 115303), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies in kind contribution.
Conflict of interest statement: RAF has received lecture fees from Teva-Aventis. RAF has not gained any remuneration from the use of the IFNβ NAb assay. PIC has received grant support from Merz Pharmaceuticals. PIC has not gained any remuneration from the use of the IFNβ NAb assay. However, Queen Mary University of London is looking to use this technology.
Contributor Information
Paul I. Creeke, Blizard Institute, Queen Mary University London, UK
Rachel A. Farrell, Department of Neuroinflammation, UCL, Institute of Neurology, Queen Square, London WC1N 3BG, UK
References
- Barnett M., Prineas J. (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55: 458–468 [DOI] [PubMed] [Google Scholar]
- Beck R., Chandler D., Cole S., Simon J., Jacobs L., Kinkel R., et al. (2002) Interferon beta-1a for early multiple sclerosis: CHAMPS trial subgroup analyses. Ann Neurol 51: 481–490 [DOI] [PubMed] [Google Scholar]
- Bendtzen K. (2003) Anti-IFN BAb and NAb antibodies: a minireview. Neurology 61: S6–S10 [DOI] [PubMed] [Google Scholar]
- Bertolotto A., Gilli F., Sala A., Capobianco M., Malucchi S., Milano E., et al. (2003) Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology 60: 634–639 [DOI] [PubMed] [Google Scholar]
- Bertolotto A., Sala A., Caldano M., Capobianco M., Malucchi S., Marnetto F., et al. (2007) Development and validation of a real time PCR-based bioassay for quantification of neutralizing antibodies against human interferon-beta. J Immunol Methods 321: 19–31 [DOI] [PubMed] [Google Scholar]
- Borden E., Sen G., Uze G., Silverman R., Ransohoff R., Foster G., et al. (2007) Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6: 975–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanet M., Radue E., Kappos L., Hartung H., Hohlfeld R., Sandberg-Wollheim M., et al. (2002) A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology 59: 1507–1517 [DOI] [PubMed] [Google Scholar]
- Clerico M., Faggiano F., Palace J., Rice G., Tintore M., Durelli L. (2008) Recombinant interferon beta or glatiramer acetate for delaying conversion of the first demyelinating event to multiple sclerosis. Cochrane Database Syst Rev (2): CD005278. [DOI] [PubMed] [Google Scholar]
- Comabella M., Vandenbroeck K. (2011) Pharmacogenomics and multiple sclerosis: moving toward individualized medicine. Curr Neurol Neurosci Rep 11: 484–491 [DOI] [PubMed] [Google Scholar]
- Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernandez O., et al. (2001) Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 357: 1576–1582 [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008) Multiple sclerosis. Lancet 372: 1502–1517 [DOI] [PubMed] [Google Scholar]
- Confavreux C., Vukusic S. (2008) The clinical epidemiology of multiple sclerosis. Neuroimaging Clin N Am 18: 589-622, ix–x [DOI] [PubMed] [Google Scholar]
- Conradt H., Egge H., Peter-Katalinic J., Reiser W., Siklosi T., Schaper K. (1987) Structure of the carbohydrate moiety of human interferon-beta secreted by a recombinant Chinese hamster ovary cell line. J Biol Chem 262: 14600–14605 [PubMed] [Google Scholar]
- Deisenhammer F., Mayringer I., Harvey J., Dilitz E., Gasse T., Stadlbauer D., et al. (2000) A comparative study of the relative bioavailability of different interferon beta preparations. Neurology 54: 2055–2060 [DOI] [PubMed] [Google Scholar]
- Deisenhammer F., Reindl M., Harvey J., Gasse T., Dilitz E., Berger T. (1999) Bioavailability of interferon beta 1b in MS patients with and without neutralizing antibodies. Neurology 52: 1239–1243 [DOI] [PubMed] [Google Scholar]
- Farrell R., Espasandin M., Lakdawala N., Creeke P., Worthington V., Giovannoni G. (2011) Incorporation of an interferon-beta neutralizing antibody assay into routine clinical practice. Mult Scler 17: 1333–1340 [DOI] [PubMed] [Google Scholar]
- Farrell R., Giovannoni G. (2007) Measuring and management of anti-interferon beta antibodies in subjects with multiple sclerosis. Mult Scler 13: 567–577 [DOI] [PubMed] [Google Scholar]
- Farrell R., Kapoor R., Leary S., Rudge P., Thompson A., Miller D., et al. (2008) Neutralizing anti-interferon beta antibodies are associated with reduced side effects and delayed impact on efficacy of Interferon-beta. Mult Scler 14: 212–218 [DOI] [PubMed] [Google Scholar]
- Farrell R., Marta M., Gaeguta A., Souslova V., Giovannoni G., Creeke P. (2012) Development of resistance to biologic therapies with reference to IFN-beta. Rheumatology (Oxford) 51: 590–599 [DOI] [PubMed] [Google Scholar]
- Fisniku L., Brex P., Altmann D., Miszkiel K., Benton C., Lanyon R., et al. (2008) Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 131: 808–817 [DOI] [PubMed] [Google Scholar]
- Francis G., Rice G., Alsop J. (2005) Interferon beta-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology 65: 48–55 [DOI] [PubMed] [Google Scholar]
- Frischer J., Bramow S., Dal-Bianco A., Lucchinetti C., Rauschka H., Schmidbauer M., et al. (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132: 1175–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilli F., Bertolotto A., Sala A., Hoffmann F., Capobianco M., Malucchi S., et al. (2004) Neutralizing antibodies against IFN-beta in multiple sclerosis: antagonization of IFN-beta mediated suppression of MMPs. Brain 127: 259–268 [DOI] [PubMed] [Google Scholar]
- Gilli F., Marnetto F., Caldano M., Sala A., Malucchi S., Capobianco M., et al. (2006) Biological markers of interferon-beta therapy: comparison among interferon-stimulated genes MxA, TRAIL and XAF-1. Mult Scler 12: 47–57 [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Barbarash O., Casset-Semanaz F., Jaber A., King J., Metz L., et al. (2007) Immunogenicity and tolerability of an investigational formulation of interferon-beta1a: 24- and 48-week interim analyses of a 2-year, single-arm, historically controlled, phase IIIb study in adults with multiple sclerosis. Clin Ther 29: 1128–1145 [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Barbarash O., Casset-Semanaz F., King J., Metz L., Pardo G., et al. (2009) Safety and immunogenicity of a new formulation of interferon beta-1a (Rebif New Formulation) in a phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler 15: 219–228 [DOI] [PubMed] [Google Scholar]
- Gneiss C., Brugger M., Millonig A., Fogdell-Hahn A., Rudzki D., Hillert J., et al. (2008) Comparative study of four different assays for the detection of binding antibodies against interferon-beta. Mult Scler 14: 830–836 [DOI] [PubMed] [Google Scholar]
- Gneiss C., Reindl M., Lutterotti A., Ehling R., Egg R., Khalil M., et al. (2004) Interferon-beta: the neutralizing antibody (NAb) titre predicts reversion to NAb negativity. Mult Scler 10: 507–510 [DOI] [PubMed] [Google Scholar]
- Goodin D., Frohman E., Hurwitz B., O'Connor P., Oger J., Reder A., et al. (2007a) Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 68: 977–984 [DOI] [PubMed] [Google Scholar]
- Goodin D., Hartung H., O'Connor P., Filippi M., Arnason B., Comi G., et al. ; BEYOND Study Group (2012) Neutralizing antibodies to interferon beta-1b multiple sclerosis: a clinico-radiographic paradox in the BEYOND trial. Mult Scler 18: 181–195 [DOI] [PubMed] [Google Scholar]
- Goodin D., Hurwitz B., Noronha A. (2007b) Neutralizing antibodies to interferon beta-1b are not associated with disease worsening in multiple sclerosis. J Int Med Res 35: 173–187 [DOI] [PubMed] [Google Scholar]
- Goodin D., Jones J., Li D., Traboulsee A., Reder A., Beckmann K., et al. (2011) Establishing long-term efficacy in chronic disease: use of recursive partitioning and propensity score adjustment to estimate outcome in MS. PLoS One 6: e22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S., Kawade Y., Kohase M., Klein J. (2001a) The neutralization of interferons by antibody. II. Neutralizing antibody unitage and its relationship to bioassay sensitivity: the tenfold reduction unit. J Interferon Cytokine Res 21: 743–755 [DOI] [PubMed] [Google Scholar]
- Grossberg S., Kawade Y., Kohase M., Yokoyama H., Finter N. (2001b) The neutralization of interferons by antibody. I. Quantitative and theoretical analyses of the neutralization reaction in different bioassay systems. J Interferon Cytokine Res 21: 729–742 [DOI] [PubMed] [Google Scholar]
- Hartung H., Munschauer F. (2004) Assessment and management of neutralizing antibodies in patients with multiple sclerosis. J Neurol 251(Suppl. 2): II40–II42 [DOI] [PubMed] [Google Scholar]
- Hesse D., Sellebjerg F., Sorensen P. (2009) Absence of MxA induction by interferon beta in patients with MS reflects complete loss of bioactivity. Neurology 73: 372–377 [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Wekerle H. (2004) Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci U S A 101(Suppl. 2): 14599–14606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien Y., Sanna A., Soderstrom M., Link H., Huang Y. (2001) Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol 121: 102–110 [DOI] [PubMed] [Google Scholar]
- Jacobs L., Beck R., Simon J., Kinkel R., Brownscheidle C., Murray T., et al. (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 343: 898–904 [DOI] [PubMed] [Google Scholar]
- Jacobs L., Cookfair D., Rudick R., Herndon R., Richert J., Salazar A., et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39: 285–294 [DOI] [PubMed] [Google Scholar]
- Kappos L., Clanet M., Sandberg-Wollheim M., Radue E., Hartung H., Hohlfeld R., et al. (2005) Neutralizing antibodies and efficacy of interferon beta-1a: a 4-year controlled study. Neurology 65: 40–47 [DOI] [PubMed] [Google Scholar]
- Kappos L., Freedman M., Polman C., Edan G., Hartung H., Miller D., et al. (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 370: 389–397 [DOI] [PubMed] [Google Scholar]
- Kob M., Harvey J., Schautzer F., Kascha S., Bibl D., Egg R., et al. (2003) A novel and rapid assay for the detection of neutralizing antibodies against interferon-beta. Mult Scler 9: 32–35 [DOI] [PubMed] [Google Scholar]
- Lam R., Farrell R., Aziz T., Gibbs E., Giovannoni G., Grossberg S., et al. (2008) Validating parameters of a luciferase reporter gene assay to measure neutralizing antibodies to IFNbeta in multiple sclerosis patients. J Immunol Methods 336: 113–118 [DOI] [PubMed] [Google Scholar]
- Lawrence N., Oger J., Aziz T., Palace J., Vincent A. (2003) A sensitive radioimmunoprecipitation assay for assessing the clinical relevance of antibodies to IFN beta. J Neurol Neurosurg Psychiatry 74: 1236–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert D., Waubant E., Burk M., Oksenberg J., Hauser S. (1996) Interferon beta-1b inhibits gelatinase secretion and in vitro migration of human T cells: a possible mechanism for treatment efficacy in multiple sclerosis. Ann Neurol 40: 846–852 [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Popescu B., Bunyan R., Moll N., Roemer S., Lassmann H., et al. (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365: 2188–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malucchi S., Gilli F., Caldano M., Sala A., Capobianco M., Di Sapio A., et al. (2011) One-year evaluation of factors affecting the biological activity of interferon beta in multiple sclerosis patients. J Neurol 258: 895–903 [DOI] [PubMed] [Google Scholar]
- Meager A., Dolman C., Dilger P., Bird C., Giovannoni G., Schellekens H., et al. (2011) An assessment of biological potency and molecular characteristics of different innovator and noninnovator interferon-beta products. J Interferon Cytokine Res 31: 383–392 [DOI] [PubMed] [Google Scholar]
- Mix E., Meyer-Rienecker H., Zettl U. (2008) Animal models of multiple sclerosis for the development and validation of novel therapies - potential and limitations. J Neurol 255(Suppl. 6): 7–14 [DOI] [PubMed] [Google Scholar]
- MS Study Group (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 43: 655–661 [DOI] [PubMed] [Google Scholar]
- MS Study Group (1996) Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neurology 47: 889–894 [DOI] [PubMed] [Google Scholar]
- Munschauer F., Kinkel R. (1997) Managing side effects of interferon-beta in patients with relapsing-remitting multiple sclerosis. Clin Ther 19: 883–893 [DOI] [PubMed] [Google Scholar]
- Pachner A., Cadavid D., Wolansky L., Skurnick J.(2009a) Effect of anti-IFN(beta) antibodies on MRI lesions of MS patients in the BECOME study. Neurology 73: 1485–1492 [DOI] [PubMed] [Google Scholar]
- Pachner A., Dail D., Pak E., Narayan K. (2005) The importance of measuring IFNbeta bioactivity: monitoring in MS patients and the effect of anti-IFNbeta antibodies. J Neuroimmunol 166: 180–188 [DOI] [PubMed] [Google Scholar]
- Pachner A., Narayan K., Price N., Hurd M., Dail D. (2003a) MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. MolDiagn 7: 17–25 [DOI] [PubMed] [Google Scholar]
- Pachner A., Oger J., Palace J. (2003b) The measurement of antibodies binding to IFNbeta in MS patients treated with IFNbeta. Neurology 61: S18–S20 [DOI] [PubMed] [Google Scholar]
- Pachner A., Warth J., Pace A., Goelz S. (2009b) Effect of neutralizing antibodies on biomarker responses to interferon beta: the INSIGHT study. Neurology 73: 1493–1500 [DOI] [PubMed] [Google Scholar]
- Paty D., Li D. (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 43: 662–667 [DOI] [PubMed] [Google Scholar]
- Platanias L. (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5: 375–386 [DOI] [PubMed] [Google Scholar]
- Polman C., Bertolotto A., Deisenhammer F., Giovannoni G., Hartung H., Hemmer B., et al. (2010) Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol 9: 740–750 [DOI] [PubMed] [Google Scholar]
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504 [PubMed] [Google Scholar]
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (2001) PRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 56: 1628–1636 [DOI] [PubMed] [Google Scholar]
- Ragonese P., Aridon P., Salemi G., D'Amelio M., Savettieri G. (2008) Mortality in multiple sclerosis: a review. Eur J Neurol 15: 123–127 [DOI] [PubMed] [Google Scholar]
- Ross C., Clemmesen K., Svenson M., Sorensen P., Koch-Henriksen N., Skovgaard G., et al. (2000) Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol 48: 706–712 [PubMed] [Google Scholar]
- Rudick R., Ransohoff R., Lee J., Peppler R., Yu M., Mathisen P., et al. (1998) In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology 50: 1294–1300 [DOI] [PubMed] [Google Scholar]
- Runkel L., Meier W., Pepinsky R., Karpusas M., Whitty A., Kimball K., et al. (1998) Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res 15: 641–649 [DOI] [PubMed] [Google Scholar]
- Sadovnick A., Ebers G. (1993) Epidemiology of multiple sclerosis: a critical overview. Can J Neurol Sci 20: 17–29 [DOI] [PubMed] [Google Scholar]
- Sharief M., Semra Y., Seidi O., Zoukos Y. (2001) Interferon-beta therapy downregulates the anti-apoptosis protein FLIP in T cells from patients with multiple sclerosis. J Neuroimmunol 120: 199–207 [DOI] [PubMed] [Google Scholar]
- Singh S. (2011) Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci 100: 354–387 [DOI] [PubMed] [Google Scholar]
- Sominanda A., Hillert J., Fogdell-Hahn A. (2008) In vivo bioactivity of interferon-beta in multiple sclerosis patients with neutralising antibodies is titre-dependent. J Neurol Neurosurg Psychiatry 79: 57–62 [DOI] [PubMed] [Google Scholar]
- Sorensen P., Deisenhammer F., Duda P., Hohlfeld R., Myhr K., Palace J., et al. (2005a) Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol 12: 817–827 [DOI] [PubMed] [Google Scholar]
- Sorensen P., Koch-Henriksen N., Bendtzen K. (2007) Are ex vivo neutralising antibodies against IFN-beta always detrimental to therapeutic efficacy in multiple sclerosis? Mult Scler 13: 616–621 [DOI] [PubMed] [Google Scholar]
- Sorensen P., Koch-Henriksen N., Ross C., Clemmesen K., Bendtzen K. (2005b) Appearance and disappearance of neutralizing antibodies during interferon-beta therapy. Neurology 65: 33–39 [DOI] [PubMed] [Google Scholar]
- Sormani M., Bonzano L., Roccatagliata L., Cutter G., Mancardi G., Bruzzi P. (2009) Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 65: 268–275 [DOI] [PubMed] [Google Scholar]
- Sospedra M., Martin R. (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23: 683–747 [DOI] [PubMed] [Google Scholar]
- Vallittu A., Halminen M., Peltoniemi J., Ilonen J., Julkunen I., Salmi A., et al. (2002) Neutralizing antibodies reduce MxA protein induction in interferon-beta-1a-treated MS patients. Neurology 58: 1786–1790 [DOI] [PubMed] [Google Scholar]
- van Beers M., Gilli F., Schellekens H., Randolph T., Jiskoot W. (2012) Immunogenicity of recombinant human interferon beta interacting with particles of glass, metal, and polystyrene. J Pharm Sci 101: 187–199 [DOI] [PubMed] [Google Scholar]
- Wandinger K., Lunemann J., Wengert O., Bellmann-Strobl J., Aktas O., Weber A., et al. (2003) TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet 361: 2036–2043 [DOI] [PubMed] [Google Scholar]
- WHO (1985) WHO Expert Committee on Biological Standardization, Thirty-fifth Report. WHO Technical Report Series. Geneva: WHO; [PubMed] [Google Scholar]
- Yong V. (2002) Differential mechanisms of action of interferon-beta and glatiramer acetate in MS. Neurology 59: 802–808 [DOI] [PubMed] [Google Scholar]
- Yong V., Chabot S., Stuve O., Williams G. (1998) Interferon beta in the treatment of multiple sclerosis: mechanisms of action. Neurology 51: 682–689 [DOI] [PubMed] [Google Scholar]