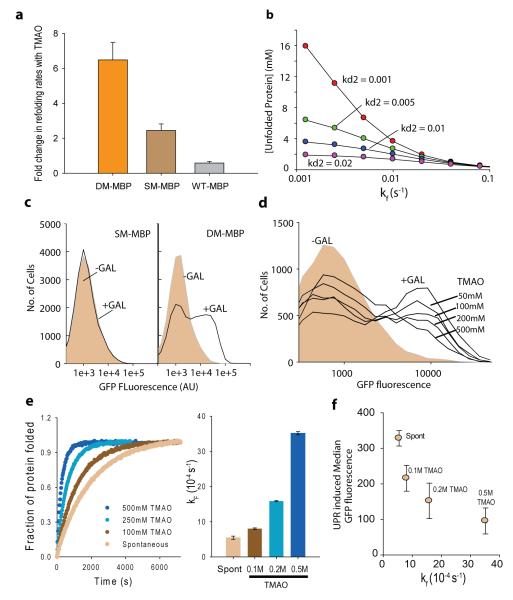
Figure 1.
Chemical chaperone assists rapid attainment of native structure. (a) Single amino acid substitution renders MBP TMAO dependent. Fold change in refolding rates of DM-MBP, SM-MBP and Wt-MBP, in presence of TMAO as compared to spontaneous refolding. The rates were obtained by fitting the curves to single exponential equations. (b) Kinetic simulation output showing steady state level of unfolded protein increasing with decreasing folding rates. Accumulation of non-native protein with varying folding rates is shown for different rates of degradation of the non-native protein. Degradation rate was varied from 0.001 to 0.02 s−1. (c) Induction of UPR as observed by increase in GFP fluorescence on Galactose induction of DM-MBP as compared to SM-MBP. GFP fluorescence was recorded using FACS. (d) UPR Induction observed by increase in GFP fluorescence on inducing DM-MBP expression in presence of different concentrations (50mM, 100mM, 200mM and 500mM) of TMAO. (e) Refolding rates of DM-MBP as a function of TMAO concentration. Effect of TMAO was measured by carrying out refolding of DM-MBP in buffer containing 100mM to 500mM TMAO. Goodness of fit is provided in Supplementary Figure 8. (f) In vitro refolding rate obtained at different concentrations of TMAO are plotted against the median UPR-induced GFP fluorescence obtained in S. cerevisiae, at the same concentrations of TMAO. All averages shown in panel a, e and f are averages of 3 independent experiments. (See also Supplementary Figure 1-10)