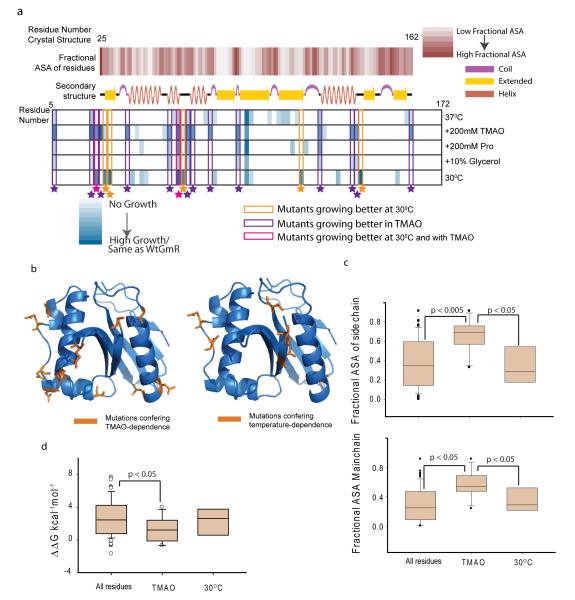
Figure3.
Analysis of TMAO assisted buffering. (a) Structural features of osmolyte and temperature sensitive mutants. First row: fractional Accessible Surface Area (ASA) of the main chain atoms of the residues calculated from the crystal structure 1bo430. Second row: secondary structure cartoon of 1bo4. Third to Seventh row: Color scale showing growth of different mutants at different conditions. Growth at 37°C(third row), in presence of TMAO at 37°C(fourth row), at 37°C in 200mM Proline (fifth row), at 37°C in Glycerol (sixth row), at 30°C (seventh row). Mutants exhibiting differential growth are enclosed in colored boxes. (b). Residues in GmR structure (1bo4) colored in orange, which when mutated to glycine-duplets lead to TMAO-dependent activity (left panel) or temperature-dependent activity (right panel). (c). Box plot showing side chain fractional ASA (top panel) and main chain fractional ASA (bottom panel) of all the residues that were mutated to obtain the library, mutants that are more active in presence of TMAO and mutants that are more active at 30°C. The significance is based on two tailed Students-t test. (d). Box plot for predicted ΔΔG (kcal−1mol−1 FU ) of all the residues mutated to obtain the library, mutants that are more active in presence of TMAO and -mutants that are more active at 30°C. Since ΔΔGFU values do not follow a normal distribution, significance was calculated using non-parametric Mann-Whitney test (see also Supplementary Figure 18,19).