Abstract
Background:
This study aimed to assess the metabolic parameters and androgen concentration in the cord blood of newborns of mothers with polycystic ovary syndrome (PCOS) in comparison with controls.
Materials and Methods:
This cross-sectional study was conducted in 2010-2011 in Isfahan, Iran. Biochemical tests were conducted on 40 infants, born from singleton pregnancies in women with PCOS and an equal number of controls.
Results:
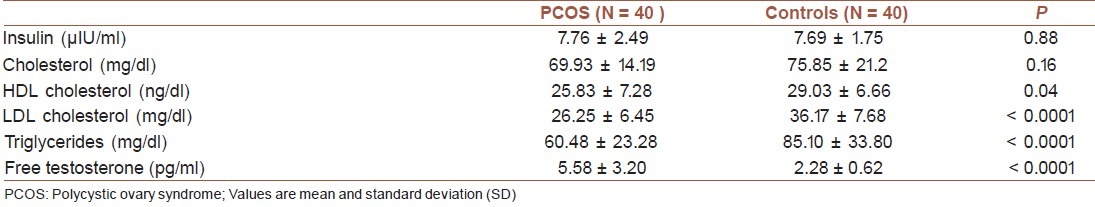
The mean weight gain during pregnancy was higher in women with PCOS than in controls (16.02 ± 4.39 vs. 9.10 ± 2.20 kg, respectively, P < 0.0001). The mean birth weight was lower in newborns of mothers with PCOS than in controls (2905.25 ± 415.59 vs. 3223.25 ± 425.02 vs. grams, respectively, P = 0.001). The mean testosterone was higher in cord blood of newborns of PCOS women than in controls (5.58 ± 3.20 vs. 2.28 ± 0.62 pg/ml, P < 0.0001). Triglycerides and LDL-C were lower in cord blood of newborns, born from PCOS women than in controls (P = 0.001). The birth weight of the newborns of PCOS mothers was negatively correlated to free testosterone of cord blood (R = -0. 26, P = 0.04).
Conclusion:
The metabolic aberration in PCOS might influence fetal birth weight and cord blood lipid profile. These disorders may be caused by an exposure to elevated testosterone level during fetal life. The offspring of PCOS women may be at higher risk for chronic diseases in later life. The clinical impact of our findings should be confirmed in future longitudinal studies.
Keywords: Androgen, metabolism, neonate, polycystic ovary syndrome, pregnancy
INTRODUCTION
Maternal hormones support fetal growth and development. There is a growing body of evidence that the endocrine, nutritional and metabolic milieu during the fetal period may have lifelong programming effects.[1–4] Therefore, it is possible to propose that newborns of the women with polycystic ovary syndrome (PCOS) are exposed to an abnormal metabolic milieu during fetal life, and consequently to chronic diseases in later life.
PCOS is one of the most common endocrine disorders among women. Based on national Institute of Health (NIH) criteria,[5] its prevalence is 7% among women of reproductive age in our community in Iran.[6]
It is suggested that an insulin resistance and compensa-tory hyperinsulinemia are key pathologic factors in PCOS.[7–10] This disorder appears to be associated with an increased risk of metabolic alterations including an insulin resistance, type II diabetes mellitus, and dyslipidemia. Thus, women with PCOS may be at risk for pregnancy complications such as gestational diabetes and hypertension.[11] An insulin may act directly and/or indirectly through the pituitary, to stimulate an ovarian androgen production. Serum levels of the androgens may be elevated. The free testosterone level is thought to be the best measure, with elevated levels found in most of PCOS patients.[12–14]
This study aimed to assess the metabolic parameters and androgen concentration in the cord blood of newborns, of mothers with PCOS in comparison with controls.
MATERIALS AND METHODS
Participants
This cross-sectional study was conducted in 2010-2011 in Isfahan, Iran. The study was approved by Research and Ethics Committee, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran (Project number:289181). Written informed consent was obtained from mothers.
The study comprised 80 mother-neonate pairs including 40 newborns from the mothers with PCOS, and an equal number of newborns from healthy mothers, referred to the same hospital.
Diagnosis of PCOS was made according to the criteria of National Institutes of Health (NIH), i.e. chronic olygomenorrhea or amenorrhea and hirsutism or serum testosterone concentration of > 0.6 ng/ml and/or free androgen index (FAI) >5.0, androstenedione concentration > 3.0 ng/ml.[5] Those patients with hyperprolactinemia, androgen – secreting neoplasms, Cushing's syndrome and late-onset 21- hydroxylase deficiency and thyroid disease were not recruited.
For control group, we selected 40 healthy women with singleton pregnancy and history of regular menstrual cycles; without hirsutism, any other manifestations of hyperandrogenism, galactorrhea, thyroid dysfunction, gestational diabetes, hypertension, and history of any chronic medication use. They were matched with the PCO group in terms of age, socio-economic levels and body mass index (BMI).
Fetal serum sex steroid and glucocorticoid levels increases under stress during labor. It is suggested that the serum lipid levels differ in during vaginal delivery from those during elective cesarean section, and it may be a consequence of elevated glucocorticoid activity.[15] Thus, we recruited mothers who underwent elective cesarean section.
Newborns with preterm delivery, malformation or genetic disorders were not included into the study.
Measures
Physical examination
All pregnant women were visited by the same gynecologist. Weight gain during pregnancy was recorded for all studied women. After delivery, physical examination of newborns was performed by a pediatrician; weight, length and head circumference were measured by calibrated instruments and under standard protocols.
Laboratory measurements
We collected umbilical mixed arterial-venous cord blood immediately after delivery, and were centrifuged, serum was kept frozen at -70°C until analysis. Serum insulin, triglycerides (TG), cholesterol, high density lipoprotein (HDL) cholesterol and low density lipoprotein cholesterol (LDL-C), total testosterone, free testosterone and dehydroepiandrosterone sulfate were measured.
Cord blood lipid profile was determined by standard colorimetric assays (Pars Azmoon, Iran), serum insulin and testosterone were determined by chemiluminescent immunoassay (DiaSorin, Saluggia, Italy).
Statistical analysis
Data were analyzed by SPSS software version 15.0 (SPSS Inc., Chicago, IL., USA). To compare the quantitative variables, the Student's t or Mann-Whitney U-tests were used, when applicable. The χ2 test was used to compare the categorical variables. An association between continuous variable was assessed by the Spearman correlation test. The significance level was set at P < 0.05.
RESULTS
The clinical characteristics of the PCOS and control groups are presented in Table 1. According to the study design, the age and the initial BMI of the mothers were not significantly different between groups. The mean weight gain during pregnancy was higher in women with PCOS than in controls (16.02 ± 4.39 vs. 9.10 ± 2.20 kg, respectively, P < 0.0001).
Table 1.
Characteristcs of pregnant women with PCOS and controls
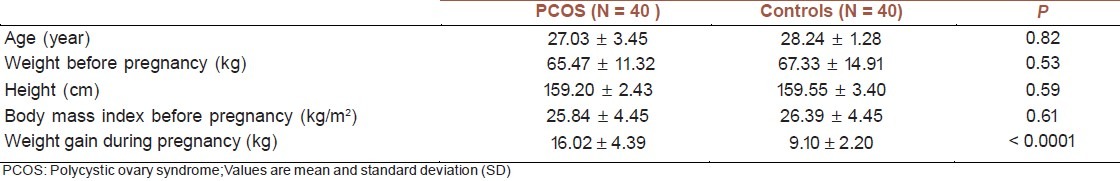
Table 2 presents the clinical characteristics of newborns of both the groups studied. The mean birth weight of newborns of mothers with PCOS was lower compared with controls (2905.25 ± 415.59 vs. 3223.25 ± 425.02 grams, respectively, P = 0.001). As presented in Table 3, mean TG and LDL-C levels were lower in cord blood of newborns born from PCOS women than in controls (P = 0.001), whereas testosterone was higher in cord blood of newborns of PCOS women than in controls (P = 0.0001).
Table 2.
Clinical characteristics of newborns of PCOS mothers and controls
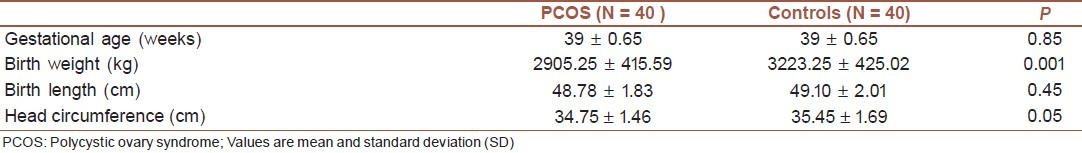
Table 3.
Metabolic parameters and androgen concentration in cord blood of PCOS mothers and controls
The birth weight of newborns of PCOS women had a weak negative correlation with mother's BMI (R = -0.29, P = 0.03), but the corresponding figure was positive in controls (R = 0.33, P = 0.01). Moreover, the birth weight in newborns of PCOS mothers was negatively correlated to free testosterone of the cord blood (R = -0.26, P = 0.04).
DISCUSSION
We found that the cord blood LDL-C and TG mean levels were significantly lower in PCOS than in control group; whereas the corresponding figure was not significantly different for the mean insulin, total-cholesterol and HDL-C.
It is well-documented that an intrauterine environment and fetal programming can have long-term impact on chronic diseases in later life.[1–4,16] In cord blood, total cholesterol level is lower than in adults, with a relatively higher proportion of HDL-C. In addition to genetic factors, ethnic differences, gestational age, fetal size, and mode of delivery influencing the composition of cord blood lipoproteins in normal pregnancies.[17–20] Fetal metabolism changes during pregnancy pathologies, for instance, while fat deposit is exaggerated in gestational diabetes, it is limited in fetal growth restriction.[21,22]
Limited experience exists about the metabolic changes in cord blood of neonates of PCOS women. The findings of our study on an alteration in the fetal lipid profile may be due to the changes in transplacental transport, which may be an appropriate physiological response to an adverse in utero environment in PCOS mothers. However, a study in Chile did not confirm the changes in the cord blood lipid profile of PCOS women.[23] The difference in the findings of these two studies might be because of the ethnic differences in cord blood lipid profile, and in part because of the mode of delivery, because we had recruited only those mothers who underwent elective cesarean section. In view of increasing evidence about fetal programming of chronic non-communicable diseases, the factors influencing cord blood lipids of PCOS mothers are suggested as an important area for further research.
In our study, androgen concentration was significantly higher in the cord blood of PCOS women than in controls. Women with PCOS have a significant increased androgen concentration, which would provide a placenta source of androgen to the fetus, and might have a long-term impact. A recent experimental study showed that elevated plasma maternal testosterone levels may cause low birth weight and then a rapid catch-up growth and hypertension in female offspring. The high testosterone level may be associated with decreased activity and expression of eNOS, which may alter endothelium-dependent vascular responses.[24] In our study, the elevated testosterone levels in cord blood of newborns of PCOS mothers may be a predictor of endothelial dysfunction and high blood pressure in the future of these infants; this hypothesis should be verified in long-term longitudinal studies.
In the present study, although weight gain during pregnancy was higher in women with PCOS than in controls, the birth weight of their offspring was significantly lower than in controls. This may be because of the fetal exposure to elevated testosterone levels, as documented by some experimental studies.[25–27]
A growing body of evidence supports the fetal programming of adult diseases and the crucial role of intrauterine growth retardation in this regard.[28–32] Therefore, the lower birth weight of PCOS women's offspring may increase the risk of chronic non-communicable diseases in later life.
The beneficial effects of metformin use during pregnancy on reducing maternal complications are documented in some studies;[33,34] however, the possible adverse effects has limited its use during pregnancy.[35] Findings of recent studies are promising for continuing metformin during pregnancy.[36,37] It can be suggested that metformin use by PCOS pregnant women may also have long-term beneficial effects for their offspring; its safety should be confirmed in longitudinal studies.
In addition to favorable effects for women, controlling PCOS may have long-term effects for their offspring in terms of primordial prevention of chronic non-communicable diseases.
Study limitations & strength
The main limitation of this study is its cross-section nature; therefore, the cause-effect cannot be assessed. The strength is the study novelty and adding information to the limited experience in this field.
CONCLUSION
The metabolic aberration and hyperandrogenemia in PCOS might influence fetal serum lipid and birth weight. These disorders may be caused by an exposure to elevated testosterone level during fetal life. The offspring of PCOS women may be at higher risk for chronic diseases in later life. The clinical impact of our findings should be confirmed in future longitudinal studies.
ACKNOWLEDGMENTS
This study was funded by Vice-Chancellery for Research, Isfahan University of Medical Sciences, Isfahan, Iran. Authors are grateful of all participants and the project team.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Paneth N, Susser M. Early origin of coronary heart disease (the “Barker hypothesis”) BMJ. 1995;310:411–2. doi: 10.1136/bmj.310.6977.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinaudo P, Wang E. Fetal Programming and Metabolic Syndrome. Annu Rev Physiol. 2012;74:107–30. doi: 10.1146/annurev-physiol-020911-153245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodyl NA, Stark MJ, Osei-Kumah A, Clifton VL. Prenatal programming of the innate immune response following in utero exposure to inflammation: a sexually dimorphic process? Expert Rev Clin Immunol. 2011;7:579–92. doi: 10.1586/eci.11.51. [DOI] [PubMed] [Google Scholar]
- 4.Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: The role of adipocytokines. Eur J Endocrinol. 2009;160:337–47. doi: 10.1530/EJE-08-0621. [DOI] [PubMed] [Google Scholar]
- 5.Artini PG, Di Berardino OM, Simi G, Papini F, Ruggiero M, Monteleone P, et al. Best methods for identification and treatment of PCOS. Minerva Ginecol. 2010;62:33–48. [PubMed] [Google Scholar]
- 6.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 7.Kosmala W, O’Moore-Sullivan TM, Plaksej R, Kuliczkowska-Plaksej J, Przewlocka-Kosmala M, Marwick TH. Subclinical impairment of left ventricular function in young obese women: Contributions of polycystic ovary disease and insulin resistance. J Clin Endocrinol Metab. 2008;93:3748–54. doi: 10.1210/jc.2008-1017. [DOI] [PubMed] [Google Scholar]
- 8.Koiou E, Dinas K, Tziomalos K, Toulis K, Kandaraki EA, Kalaitzakis E, et al. The phenotypes of polycystic ovary syndrome defined by the 1990 diagnostic criteria are associated with higher serum vaspin levels than the phenotypes introduced by the 2003 criteria. Obes Facts. 2011;4:145–50. doi: 10.1159/000327935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupisti S, Kajaia N, Dittrich R, Duezenli H, Beckmann M W, Mueller A. Body mass index and ovarian function are associated with endocrine and metabolic abnormalities in women with hyperandrogenic syndrome. Eur J Endocrinol. 2008;158:711–9. doi: 10.1530/EJE-07-0515. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, et al. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2008;69:634–41. doi: 10.1111/j.1365-2265.2008.03247.x. [DOI] [PubMed] [Google Scholar]
- 11.Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106:131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 12.Huang A, Brennan K, Azziz R. Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril. 2010;93:1938–41. doi: 10.1016/j.fertnstert.2008.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pall ME, Lao MC, Patel SS, Lee ML, Ghods DE, Chandler DW, et al. Testosterone and bioavailable testosterone help to distinguish between mild Cushing's syndrome and polycystic ovarian syndrome. Horm Metab Res. 2008;40:813–8. doi: 10.1055/s-0028-1087186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puurunen J, Piltonen T, Jaakkola P, Ruokonen A, Morin-Papunen L, Tapanainen JS. Adrenal androgen production capacity remains high up to menopause in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1973–8. doi: 10.1210/jc.2008-2583. [DOI] [PubMed] [Google Scholar]
- 15.Schulpis KH, Margeli A, Akalestos A, Vlachos GD, Partsinevelos GA, Papastamataki M, et al. Effects of mode of delivery on maternal-neonatal plasma antioxidant status and on protein S100B serum concentrations. Scand J Clin Lab Invest. 2006;66:733–42. doi: 10.1080/00365510600977737. [DOI] [PubMed] [Google Scholar]
- 16.Wells JC. Historical cohort studies and the early origins of disease hypothesis: Making sense of the evidence. Proc Nutr Soc. 2009;68:179–88. doi: 10.1017/S0029665109001086. [DOI] [PubMed] [Google Scholar]
- 17.Woollett LA. Review: Transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32(Suppl 2):S218–21. doi: 10.1016/j.placenta.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelishadi R, Badiee Z, Adeli K. Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatr Perinat Epidemiol. 2007;21:518–24. doi: 10.1111/j.1365-3016.2007.00870.x. [DOI] [PubMed] [Google Scholar]
- 19.Badiee Z, Kelishadi R. Cord blood lipid profile in a population of Iranian term newborns. Pediatr Cardiol. 2008;29:574–9. doi: 10.1007/s00246-007-9149-0. [DOI] [PubMed] [Google Scholar]
- 20.Kharb S, Kaur R, Singh V, Sangwan K. Birth weight, cord blood lipoprotein and apolipoprotein levels in Indian newborns. Int J Prev Med. 2010;1:29–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Desoye G, Gauster M, Wadsack C. Placental transport in pregnancy pathologies. Am J Clin Nutr. 2011;94(6 Suppl):1896S–902S. doi: 10.3945/ajcn.110.000851. [DOI] [PubMed] [Google Scholar]
- 22.Rebholz SL, Burke KT, Yang Q, Tso P, Woollett LA. Dietary fat impacts fetal growth and metabolism: Uptake of chylomicron remnant core lipids by the placenta. Am J Physiol Endocrinol Metab. 2011;301:E416–25. doi: 10.1152/ajpendo.00619.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maliqueo M, Echiburú B, Crisosto N, Amigo P, Aranda P, Sánchez F, et al. Metabolic parameters in cord blood of newborns of women with polycystic ovary syndrome. Fertil Steril. 2009;92:277–82. doi: 10.1016/j.fertnstert.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev. 2011;87:407–14. doi: 10.1016/j.earlhumdev.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–8. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 26.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–93. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 27.Crespi EJ, Steckler TL, Mohankumar PS, Padmanabhan V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. J Physiol. 2006;572:119–30. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberle C. [Fetal programming of type 2 diabetes--intrauterine growth retardation (IUGR) as risk factor?] MMW Fortschr Med. 2010;152(Suppl 3):76–82. [PubMed] [Google Scholar]
- 29.Remacle C, Bieswal F, Bol V, Reusens B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am J Clin Nutr. 2011;94(6 Suppl):1846S–52S. doi: 10.3945/ajcn.110.001651. [DOI] [PubMed] [Google Scholar]
- 30.Sebert S, Sharkey D, Budge H, Symonds ME. The early programming of metabolic health: is epigenetic setting the missing link? Am J Clin Nutr. 2011;94(6 Suppl):1953S–8S. doi: 10.3945/ajcn.110.001040. [DOI] [PubMed] [Google Scholar]
- 31.Achard V, Boullu-Ciocca S, Desbriére R, Grino M. Perinatal programming of central obesity and the metabolic syndrome: Role of glucocorticoids. Metab Syndr Relat Disord. 2006;4:129–37. doi: 10.1089/met.2006.4.129. [DOI] [PubMed] [Google Scholar]
- 32.Simmons RA. Role of metabolic programming in the pathogenesis of beta-cell failure in postnatal life. Rev Endocr Metab Disord. 2007;8:95–104. doi: 10.1007/s11154-007-9045-1. [DOI] [PubMed] [Google Scholar]
- 33.Kovo M, Weissman A, Gur D, Levran D, Rotmensch S, Glezerman M. Neonatal outcome in polycystic ovarian syndrome patients treated with metformin during pregnancy. J Matern Fetal Neonatal Med. 2006;19:415–9. doi: 10.1080/14767050600682370. [DOI] [PubMed] [Google Scholar]
- 34.Nawaz FH, Khalid R, Naru T, Rizvi J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res. 2008;34:832–7. doi: 10.1111/j.1447-0756.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 35.Bolton S, Cleary B, Walsh J, Dempsey E, Turner MJ. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur J Pediatr. 2009;168:203–6. doi: 10.1007/s00431-008-0737-7. [DOI] [PubMed] [Google Scholar]
- 36.Khattab S, Mohsen IA, Aboul Foutouh I, Ashmawi HS, Mohsen MN, van Wely M, et al. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol Endocrinol. 2011;27:789–93. doi: 10.3109/09513590.2010.540600. [DOI] [PubMed] [Google Scholar]
- 37.De Leo V, Musacchio MC, Piomboni P, Di Sabatino A, Morgante G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol. 2011;157:63–6. doi: 10.1016/j.ejogrb.2011.03.024. [DOI] [PubMed] [Google Scholar]


