Abstract
The concept of targeting cancer therapeutics towards specific mutations or abnormalities in tumor cells which are not found in normal tissues has the potential advantages of high selectivity for the tumor and correspondingly low secondary toxicities. Many human malignancies display activating mutations in the Ras family of signal-transducing genes or over-activity of p21Ras-signaling pathways. Carcinoid and other neuroendocrine tumors similarly have been demonstrated to have activation of Ras signaling directly by mutations in Ras, indirectly by loss of Ras-regulatory proteins, or via constitutive activation of upstream or downstream effector pathways of Ras, such as growth factor receptors or PI3-Kinase and Raf/MAP kinases. We previously reported that aberrant activation of Ras signaling sensitizes cells to apoptosis when the activity of the PKCδ isozyme is suppressed, and that PKCδ suppression is not toxic to cells with normal levels of p21Ras signaling. We demonstrate here that inhibition of PKCδ by a number of independent means, including genetic mechanisms (shRNA) or small molecule inhibitors, is able to efficiently and selectively repress the growth of human neuroendocrine cell lines derived from bronchopulmonary, foregut or hindgut tumors. PKCδ inhibition in these tumors also efficiently induced apoptosis. Exposure to small-molecule inhibitors of PKCδ over a period of 24 hr is sufficient to significantly suppress cell growth and clonogenic capacity of these tumor cell lines.
Neuroendocrine tumors are typically refractory to conventional therapeutic approaches. This Ras-targeted therapeutic approach, mediated through PKCδ suppression, which selectively takes advantage of the very oncogenic mutations which contribute to the malignancy of the tumor, may hold potential as a novel therapeutic modality.
Keywords: carcinoid, Ras, apoptosis, cancer
Introduction
The concept of targeting cancer therapeutics towards specific mutations or abnormalities in tumor cells which are not found in normal tissues has the potential advantages of high selectivity for the tumor and correspondingly low secondary toxicities. At least 30% of all human malignancies display activating mutations in the RAS genes, and perhaps another 60% display other activating mutations in, or over-activity of, p21Ras-signaling pathways. We previously reported that aberrant activation of Ras results in an absolute dependency upon PKCδ-mediated survival pathways (Xia et al. 2007; Xia et al. 2009). Over-activity of p21Ras signaling therefore sensitizes tumor cells to apoptosis induced by suppression of PKCδ activity, whereas suppression of PKCδ activity is not toxic to cells with normal levels of p21Ras activity or signaling (Chen & Faller 1995; Xia et al. 2007; Chen & Faller 1996; Chen et al. 1998a; Chen et al. 1998b; Chen et al. 2001; Chen et al. 2003; Liou et al. 2000; Liou et al. 2004). We have shown that this tumor-specific susceptibility, designated “Ras-mediated apoptosis,” can be exploited as a targeted cancer therapeutic.
Bronchopulmonary, gastrointestinal and pancreatic neuroendocrine tumors are rare tumors originating from neuroendocrine tissues (Oberg 1999). Clinical symptoms are often caused by the production of hormonally-active substances by the tumor such as serotonin, gastrin, insulin, vasoactive intestinal peptide, pancreatic polypeptide, or substance P. Chromogranin A is produced by 80–100% of neuroendocrine tumors and serves as a reliable biochemical marker. The disease can be cured by early surgery, but the vast majority of tumors have metastases at the time of diagnosis, which makes palliation the cornerstone of management. Debulking surgery, liver artery embolization, and chemotherapy aim at tumor mass reduction, whereas somatostatin analogues and IFN are used for control of symptoms (Arnold et al. 2000; Frank et al. 1999). Radioactively-labeled somatostatin analogues have been used in trials, with response rates ~30% (Arnold et al. 2002). Response rates of cytoreductive approaches are generally below 60%, however, and long-term responses are not maintained (Oberg 2001). New and more effective approaches are therefore needed in the treatment of neuroendocrine malignancies.
Carcinoid and other neuroendocrine tumors of the gastrointestinal tract share a number of the same genetic abnormalities (deletions and mutations) as adenocarcinomas (Leotlela et al. 2003; Arber et al. 1997). These abnormalities include activation of Ras signaling directly by mutations in the Ras protein, indirectly by loss of Ras-regulatory proteins such as NF-1, or via constitutive activation of Ras-linked growth factor receptors, or downstream effector pathways of Ras, such as PI3K and Raf/MAP kinases. For example, activation of H-Ras and Ki-Ras signaling is detected in a significant fraction of carcinoid and other gastrointestinal neuroendocrine tumors (65% and 10%, respectively) (Liedke et al. 1998; Maitra et al. 2000). Ras itself can be activated in neuroendocrine tumors by point mutation or by loss of regulators of Ras, such as RassF1A or NF-1 (Liu et al. 2005; Stancu et al. 2003; Bausch et al. 2007). The Raf/mitogen-activated protein kinase (Raf/MAP kinase), or the MAP kinases directly downstream of Raf, are frequently activated in neuroendocrine tumors (Tannapfel et al. 2005; Karhoff et al. 2007; Perren et al. 2004; Kunnimalaiyaan & Chen 2006). The PI3K pathway can be activated in neuroendocrine tumors from deletion of the tumor suppressor gene PTEN (phosphatase and tensin homologue). Loss of PTEN in neuroendocrine tumors increases in frequency with the loss of differentiation in the tumor (Wang et al. 2005), and loss of PTEN expression may represent an important step in the progression of neuroendocrine tumors (Wang et al. 2002).
We demonstrate in this report that human neuroendocrine tumor cell lines of pulmonary and gastrointestinal origin are sensitive to PKCδ inhibition. Knockdown of PKCδ by exposure to PKCδ–specific shRNA, or suppression of PKCδ activity by diverse small-molecule inhibitors, is sufficient to inhibit proliferation of these human neuroendocrine tumor cell lines and efficiently induce apoptosis.
Materials and Methods
Cell Lines
BON1, a human foregut (pancreatic) carcinoid tumor cell line (Parekh et al. 1994) was obtained from Kjell Oberg (Uppsala University, Sweden) through Dr. Evan Vosburgh. H727 cells, derived from a human bronchopulmonary carcinoid tumor (Schuller et al. 1987), were purchased from ATCC (Manassas, VA). The CNDT 2.5 cell line, initially described as a human midgut carcinoid tumor cell line (Van Buren et al. 2007), was provided by Dr. Lee Ellis, (MD Anderson Cancer Center). The provenance of this cell line is currently under review by the originator. NIH-3T3 and NIH-Ras cells have been previously described (Xia et al. 2009; Xia et al. 2007). MCF10 cells, BxPC3 cells and PZ-HPV-7 cells were obtained from ATCC. BON1, H727, CNDT 2.5 cells were propagated in 10% fetal bovine serum (Invitrogen); Dulbecco’s Modification of Earle’s Media/Hams F-12 50:50 media (Cellgro); 2 mM L-Glutamine (Invitrogen); 200 U Penicillin/ml; 200 μg Streptomycin/ml (Invitrogen); 10 ng/ml Nerve Growth Factor (Invitrogen); 1 x MEM Non-Essential Amino Acids (Cellgro); 1 x MEM Vitamin Solution (Cellgro); 1 mM Sodium Pyruvate; 0.015 M HEPES buffer (pH7.3) (American Bioanalytical). NIH-3T3, NIH-Ras, MCF10, MCF1-Ras, BxPC3, and PZ-HPV-7cells were propagated in 10% fetal bovine serum (Invitrogen); Dulbecco’s Modification of Earle’s Media (Cellgro); 2 mM L-Glutamine (Invitrogen); 200 U Penicillin/ml; 200 ug Streptomycin/ml (Invitrogen).
Clonogenic Assays
100,000 cells were seeded on 100 mm dishes with 10 ml media per dish (Li et al. 2004). On day 4, cells were treated with a PKCδ inhibitor, or vehicle control for either 6, 18, 24 or 48 hours. Cells were trypsinized; counted via the trypan blue exclusion method in order to determine the number of live cells in the sample, and 500 live cells were seeded in triplicate onto 6 well plates. Cells were monitored for appropriate colony size and re-fed every three to four days. At Day 17, cells were stained with ethidium bromide (Guda et al. 2007) and counted using UVP LabWorks software.
PKC Kinase Activity Assays
Assays were carried out using recombinant PKCα or PKCδ, (Invitrogen) and the Omnia® Kinase Assays (Invitrogen) with a “PKC-kinase-specific” peptide substrate. Incorporation of a chelation-enhanced fluorophore (CHEF) results in an increase in fluorescence (λex360/λem485) upon phosphorylation. The kit was used according to the manufacturer’s instructions.
Reagents
Rottlerin was purchased from (EMD Biosciences). The PKCδ inhibitor KAM1 is a chimeric molecule combining the chromene portion of rottlerin with the carbazole portion of staurosporine.
Cell proliferation assays
Cell proliferation was assessed using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Roche, Mannheim, Germany). The number of viable cells growing in a single well on a 96-well microtiter plate was estimated by adding 10 μl of MTT solution (5 mg/ml in phosphate-buffered saline [PBS]). After 4 h of incubation at 37°C, the stain is diluted with 100 μl of dimethyl sulfoxide. The optical densities are quantified at a test wavelength of 570 nm and a reference wavelength of 690 nm on a multiwell spectrophotometer. In some assays, MTS was used as substrate (Promega, Madison, WI), and the absorbance of the product was monitored at 490 nm. Cell enumeration was carried out using a hemocytometer, and viable cells identified by trypan blue exclusion.
Cytotoxicity Assay
LDH release was assessed by spectrophotometrically measuring the oxidation of NADH in both the cells and media. Cells were seeded in 24-well plates, and exposed to PKCδ inhibitors or vehicle. After different times of exposure, cytotoxicity was quantified by a standard measurement of LDH release with the use of the LDH assay kit (Roche Molecular Biochemicals) according to the manufacturer’s protocol. Briefly, total culture medium was cleared by centrifugation. For assay of released LDH, supernatants were collected. To assess total LDH in cells, Triton X-100 was added to vehicle (control) wells to release intracellular LDH. LDH assay reagent was added to lysates or supernatants and incubated for up to 30 min at room temperature in dark, the reaction was stopped, and the absorbance was measured at 490 nm. The percentage of LDH release was then calculated as the LDH in the supernatants as a fraction of the total LDH.
Immunoblot Analyses
Levels of proteins were measured and quantitated in carcinoid cell lines, as we have previously reported (Xia et al. 2007). Harvested cells were disrupted in a buffer containing 20 mM Tris (pH 7.4), 0.5% NP-40, and 250 mM NaCl. Total protein (40 μg) is separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes or PVDF membranes. Membranes are blocked overnight and probed with affinity-purified antibodies against PKCα and δ (BD Transduction Lab), or β-actin (Sigma). After washing, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized using the Amersham enhanced chemiluminescence ECL system, and quantitated by digital densitometry. Antibodies against human ERK, phospho-ERK, AKT and phospho-Ser473-AKT were purchased from Cell Signaling (Danvers, MA). GTP-bound Ras was assayed by affinity purification using a Raf-1/RBD agarose conjugate (Upstate Biotechnology, Lake Placid, NY), and detected with a pan-Ras antibody (Cell Signaling, Danvers, MA), following the manufacturer’s instructions.
Down-regulation of PKCδ by shRNA and lentiviral vectors
shRNA knockdown of PKCδ and PKCα shRNA duplexes for PKCδ (shRNAs) are obtained from Qiagen (Valencia, Ca). The shRNA sequences for targeting PKCδ are PKCδ-shRNA-1 (5′-GAUGAAGGAGGCGCUCAGTT-3′) and PKCδ shRNA-2 (5′-GGCUGAGUUCUGGCUGGA-CTT-3′) (32). The corresponding scrambled shRNAs were used as negative control. These shRNA sequences were also cloned into the pRNA6.1-Neo vector with a GFP tag according to the manufacturer’s instructions (GenScript, Piscataway, NJ). shRNA for PKCα (PKC-PKCα-V6) are purchased from Upstate (Lake Placid, NY). Transfection of shRNA (oligo) is performed using 50 nM PKCδ shRNA, or the same amount of scrambled shRNA and Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Transfection of plasmid-based shRNA vectors are carried out using the same method. PKCδ protein levels were determined by immunoblot analysis (see below). The lentiviral vectors were previously described (Xia et al. 2009).
Statistical analysis
Experiments were carried out in triplicate for all experimental conditions. Data are shown as mean ± SD. Where applicable, a two-tailed Student’s t test or ANOVA was performed on the means of two sets of sample data and considered significant if p ≤ 0.05.
Results
PKCδ depletion by shRNA inhibits proliferation and induces cytotoxicity in human neuroendocrine cell lines
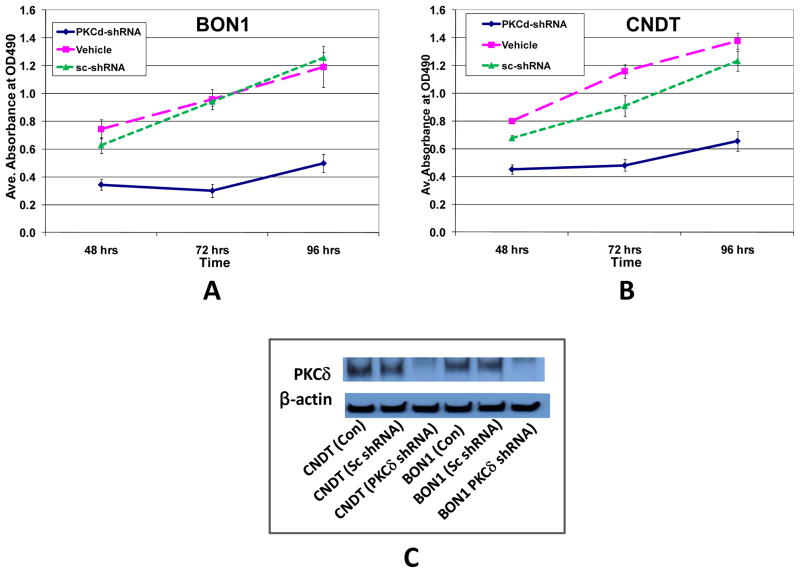
To determine the effects of specific PKCδ depletion on the proliferation and survival of human neuroendocrine tumor cell lines, PKCδ-specific shRNA was used to knock-down PKCδ mRNA/protein. Cell lines studied for sensitivity included BON1, a human foregut (pancreatic) carcinoid tumor cell line; H727 cells, derived from a human bronchopulmonary carcinoid tumor; and the CNDT 2.5 cell line, a human cell line with neuroendocrine markers, initially described as a human midgut carcinoid tumor cell line. Exposure of the BON1 and CNDT cell lines to PKCδ-specific shRNA in culture resulted in a profound inhibition of proliferation (Figs. 1A&B). In contrast, exposure of the same cells to a control (scrambled shRNA) did not affect proliferation. Efficient knockdown of PKCδ protein by specific shRNA was verified by immunoblotting.
Figure 1. Effects of PKCδ knockdown by shRNA on proliferation of human neuroendocrine tumor BON1 and CNDT cells.
BON1 (A) and CNDT 2.5 (B) cells were grown to 50% confluence in 96-well plates and then treated with PKCδ-shRNA or scrambled shRNA (sc-shRNA). The corresponding solvent equivalent volumes were used as vehicle controls (Vehicle). After 48, 72, and 96 hours of treatment, cell number was evaluated by MTS assay. (C) Immunoblot analysis of PKCδ protein levels in the same cell lines 72 hr after exposure to lentivirus-transfected PKCδ-targeting shRNA or scrambled shRNA. Lentiviral PKCδ-targeted shRNA efficiently inhibited PKCδ protein expression.
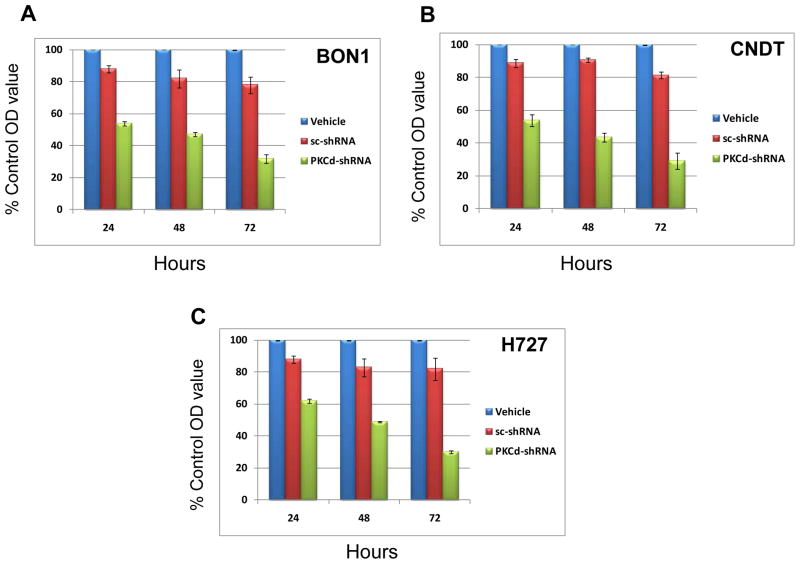
To confirm and extend these experiments, lentiviral vectors containing the same shRNA sequences (PKCδ-specific or scrambled) were constructed. Infection of the BON1, H727 and CNDT cell lines with these vectors demonstrated PKCδ-specific inhibition of proliferation (Fig. 2A–C). The lentiviral vector containing the scrambled sequence (control) consistently had a modest inhibitory effect on proliferation of both cell lines, but this never reached statistical significance. Efficient knockdown of PKCδ protein by the specific shRNA was verified by immunoblotting.
Figure 2. Effects of PKCδ knockdown by PKCδ-specific shRNA lentivirus on proliferation of human neuroendocrine tumor cells.
BON1 (A), CNDT 2.5 (B) and H727 (C) cells were grown to 50% confluence in 96-well plates and then infected with PKCδ-shRNA-Lentivirus (PKCδ-shRNA) or scrambled shRNA Lentivirus (sc-shRNA). Cell exposed to mock lentiviral infection (Vehicle) also served as controls. After 24, 48, and 72 hours of treatment, cell proliferation was evaluated by MTS assay. Control values were normalized to 100%. Error bars represent SEM. P values for comparison between control (scrambled shRNA) lentivirus and PKCδ-shRNA lentivirus effects on cell number reached significance at 24 hr of exposure (p ≤ 0.001) for all cell lines, and remained significant at the 48 and 72 hr time points.
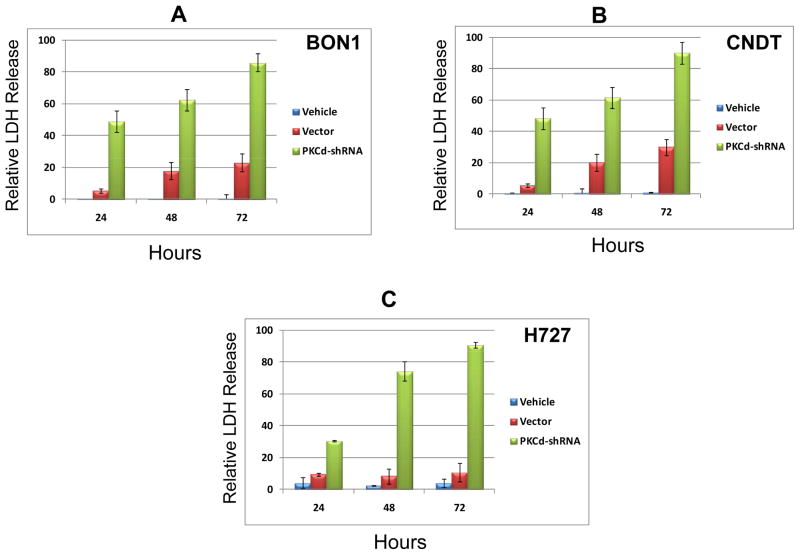
To determine if the inhibition of tumor cell proliferation by PKCδ knockdown was accompanied by cytotoxic effects on the tumor cells, cytotoxicity in these cell lines was evaluated by quantitating LDH release. Lactose dehydrogenase (LDH), a stable cytoplasmic enzyme, is rapidly released into the cell culture medium after damage of the plasma membrane, and its level correlates quantitatively with the extent of cytotoxicity. Significant increases in LDH release / cytotoxicity were detected within 24 hr of exposure to the lentiviral vector containing the PKCδ shRNA, and this release increased to approach the maximum possible LDH release (complete cell lysis, positive control) by 72 hr (Fig. 3A–C). Only modest, but detectable, increases in LDH release were induced by the control (scrambled shRNA) lentiviral vector.
Figure 3. Cytotoxic effects of PKCδ-knockdown by shRNA-lentivirus on human neuroendocrine tumor cell lines.
BON1 (A), CNDT 2.5 (B) and H727 (C) cells were grown to 50% confluence in 96-well plates and then infected with PKCδ-shRNA-lentivirus or scrambled shRNA lentivirus (Vector). Cell exposed to mock lentiviral infection (Vehicle) also served as controls. After 24, 48 and 72 hr of treatment, cell cytotoxicity was evaluated by LDH-release assay. Total maximal LDH release was assigned the arbitrary value of 100%. Error bars represent SEM. P values for comparison between control (scrambled shRNA) lentivirus and PKCδ-shRNA lentivirus effects on LDH release reached significance at 24 hr of exposure (p ≤ 0.004) for all cell lines, and remained significant at the 48 and 72 hr time points.
Small molecule inhibitors of PKCδ are cytotoxic to neuroendocrine tumor cell lines
We next determined whether a series of small-molecule PKCδ inhibitors would inhibit the growth of human neuroendocrine tumor cell lines. While not as specific for the PKCδ isozyme as technology employing genetic knockdown of the PKCδ mRNA and protein, such small-molecule inhibitors are more relevant for eventual therapeutic application.
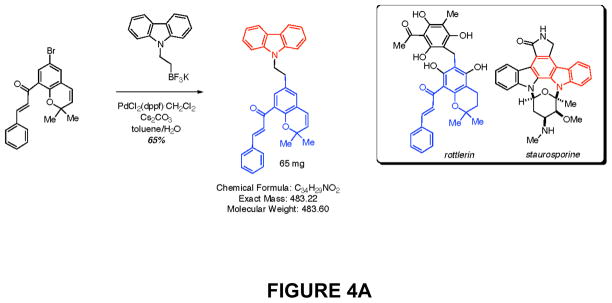
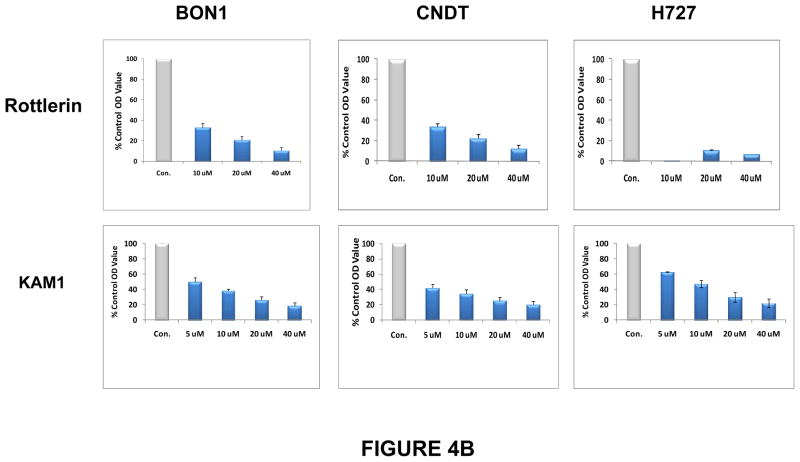
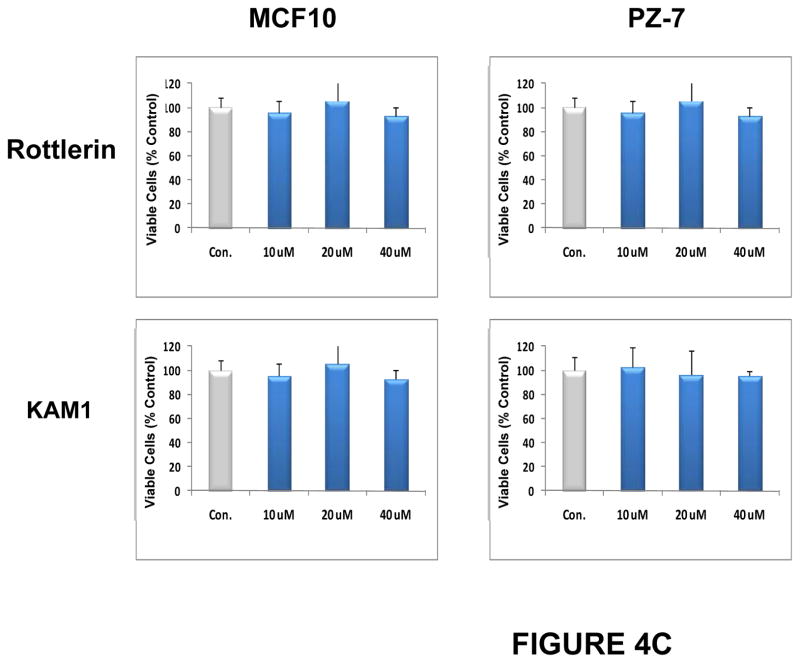
Rottlerin is a naturally-occurring product (Fig. 4A)which inhibits purified PKCδ at an IC50 of 0.2–3.0 μM in vitro, and inhibits PKCδ in cultured cells with an IC50 of 5 μM in vivo (data not shown). It is relatively selective for PKCδ (PKCδ IC50/PKCα IC50 > 30:1) (Gschwendt et al. 1994; Kikkawa et al. 2002), and this relative selectivity was confirmed in our in vitro assays (not shown). Furthermore, this compound not only directly inhibits purified PKCδ, but also, over longer periods of exposure, significantly down-regulates PKCδ protein specifically in cells, while having no effect on the levels of other PKC isozymes (Xia et al. 2007). Exposure to rottlerin produced a dose- and time-dependent decrease in cell number in the BON1, the CNDT 2.5, and the H727 cell lines, with an IC50 of approximately 5 μM, by 48 hr (not shown), and a significant reduction in relative cell numbers by 72 hr (Fig. 4B). In contrast, rottlerin had no significant effect on the growth of two non-transformed human cell lines, MCF10 (breast epithelial cells) and PZ-HPV-7 (prostate epithelial cells) (Fig. 4C). In addition, we have previously demonstrated that exposure to rottlerin under these same culture conditions has no significant effect on the growth of a number of other non-tumorigenic murine or human cells or cell lines (Xia et al. 2007).
Figure 4. Effects of PKCδ inhibitors on proliferation of human neuroendocrine tumor cell lines.
(A) Structures of rottlerin and KAM1, a rottlerin/staurosporine chimera. Neuroendocrine tumor cell lines (B) or non-transformed human cell lines MCF10 or PZ-HPV-7 (PZ-7) (C) were grown to 80% confluence in 96-well plates and then exposed to rottlerin or KAM1 at 5, 10, 20 or 40 μM. The corresponding equivalent volumes of solvent (DMSO) were used as vehicle controls (Con). After 72 hr of exposure, cell growth was evaluated by MTT assay or enumeration of viable cells. Control values were normalized to 100%. P values for comparison between control vehicle and rottlerin effects on cell number in the neuroendocrine tumor cell lines reached significance at 24 hr of exposure (p ≤ 0.004) for all cell lines, and remained significant at the 48 and 72 hr time points. P values for comparison between control vehicle and KAM1 effects on cell number in the neuroendocrine tumor cell lines reached significance by 72 hr of exposure (p ≤ 0.02) for all cell lines. In contrast, in the non-transformed human cell lines, neither exposure to rottlerin or KAM1 produced a statistically-significant effect on cell proliferation.
Docking studies were conducted to predict how rottlerin binds to PKCδ. Rottlerin was docked into the catalytic binding site of several different PKC crystal structures. The structure of PKCθ complexed with staurosporine (pdb code 1XJD) was selected as the most suitable model. It is known from crystal structures of many kinase/inhibitor complexes that the kinase active site is flexible; therefore, regions known to be flexible were allowed to be free during the docking procedures. Chimeric molecules were designed using the PKCδ model developed from the rottlerin docking studies. The strategy was to retain most of the chromene part of rottlerin, which is assumed to give rottlerin its specificity but to vary the “head group” which is assumed to bind to the hinge region of the kinase active site. A novel PKCδ inhibitor, KAM1, which is a chimeric molecule containing the substituted chromene portion of rottlerin and the N-alkylated carbazole portion of staurosporine (a non-selective pan-PKC inhibitor) (Fig. 4A), was next tested for cytotoxic effects on neuroendocrine tumor cells. Comparative analyses of PKCδ-inhibitory activity demonstrated an in vitro IC50 of 0.2 μM for rottlerin and an IC50 of 0.9 μM for KAM1. In contrast, the PKCα IC50 was greater than 50 μM for each compound, demonstrating some specificity for the novel isozyme PKCδ over classic isozyme PKCα. KAM1 produced a dose- and time-dependent decrease in cell number in the BON1, the CNDT 2.5, and the H727 cell lines, with an in vivo IC50 of approximately 12 μM, by 48 hr (not shown), and an 80% reduction in cell numbers by 72 hr at the highest concentrations tested (Figs. 4A&B).
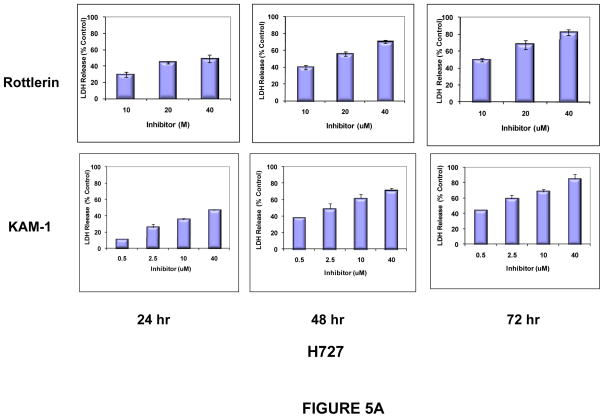
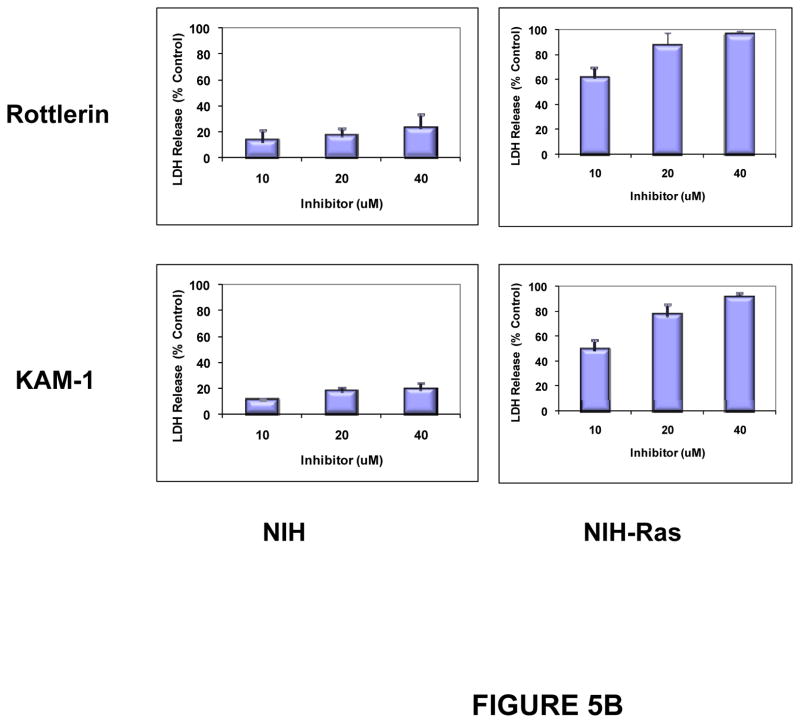
In parallel, cytotoxicity, as assessed by LDH release, was induced by exposure of the three carcinoid cell lines to rottlerin and to KAM1. In all three cell lines, cytotoxicity increased as a function of time and concentration of these inhibitors (Fig. 5A). As controls for the targeted nature of this approach, LDH release was assayed in NIH-3T3 cells (a non-transformed murine mesenchymal cell line, and in NIH-3T3 cells transformed by the introduction of an activated Ha-Ras allele (NIH-Ras). Consistent with previous reports, significant susceptibility to cytotoxicity after exposure to these PKCδ inhibitors was conferred in NIH cells by the presence of an activated Ras protein (Fig. 5B).
Figure 5. Cytotoxic effects of PKCδ-inhibitors on human neuroendocrine tumor cell lines.
H727 cells (A) or NIH and NIH-Ras cells (B) were grown to 50% confluence in 96-well plates and then exposed to Rottlerin or KAM1 at the concentrations indicated. After 24, 48 and 72 hr of exposure, cell cytotoxicity was evaluated by LDH-release assay. Only the 72 hr time point is shown for the NIH and NIH-Ras cells (panel B). Cells exposed to vehicle alone served as controls for determination of baseline LDH release values, which were subtracted at each time point. Total maximal LDH release was assigned the arbitrary value of 100%. Error bars represent SEM. P values for comparison between control (vehicle) and Rottlerin or KAM1 effects on LDH release reached significance by 24 hr of exposure (p ≤ 0.003) for the H727 and NIH-Ras cells, and remained significant at the 48 and 72 hr time points. In contrast, there were no statistically-significant changes in LDH release for the NIH cells exposed to either compound, compared to control.
Ras signaling in neuroendocrine tumor cell lines
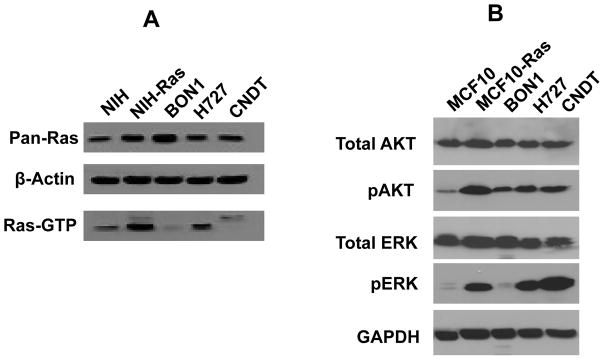
Because of their sensitivity to PKCδ inhibition and “Ras-mediated apoptosis,” the activity of p21Ras protein in these neuroendocrine tumor cell lines was assessed by affinity pull-down of GTP-bound p21Ras species. Endogenous Ras activity (GTP-bound Ras) was high in the H727 cells, and was not evident in the CNDT or BON1 cells lines, which contained GTP-bound p21Ras levels comparable to those found in non-transformed cells (Fig. 6A).
Fig 6. Ras signaling in neuroendocrine tumor cell lines.
A. p21Ras activity in neuroendocrine tumor cell lines. Nuclear-free lysates from NIH/3T3 cells (negative control), NIH/3T3-Ras cells (positive control), BON1 cells, H727 cells, and CNDT cells, containing a total of 400 μg of protein from each indicated cell type, were used for analysis of Ras activity by Raf-RBD pull-down of GTP-bound p21Ras. Equal loading was demonstrated by re-probing the blot with anti-β-actin antibody. Pan-p21Ras protein expression levels were also analyzed.
B. Activation of Ras signaling pathways in neuroendocrine tumor cell lines. Cell lysates from MCF10 cells (negative-control), MCF10-Ras cells (positive control), BON1 cells, H727 cells, and CNDT cells were separated by SDS-polyacrylamide gel electrophoresis, transferred to a membrane, and immunoblotted with antibodies against ERK, phospho-ERK, AKT, phospho-Thr308 AKT, and GAPDH (as a loading control).
It has been previously demonstrated that aberrant activation of certain Ras signaling pathways, including the PI3K-AKT pathway and the Raf-MAPK pathway, are sufficient to render tumor cells susceptible to PKCδ inhibition, even in the absence of activating mutations of Ras itself (Xia et al. 2007). The activation status of downstream elements of these signaling pathways was therefore explored in these neuroendocrine tumor cell lines. Evidence for activation of Raf-MAPK, as defined by relative elevation of phospho-ERK levels, was observed in the H727 and CNDT lines (compared to the non-transformed negative-control cell line MCF10) (Fig. 6B). Evidence for some activation of PI3K signaling, as defined by activating phosphorylation of AKT (Ser473) relative to the non-transformed negative control cell line MCF10, was observed in all three neuroendocrine tumor cell lines.
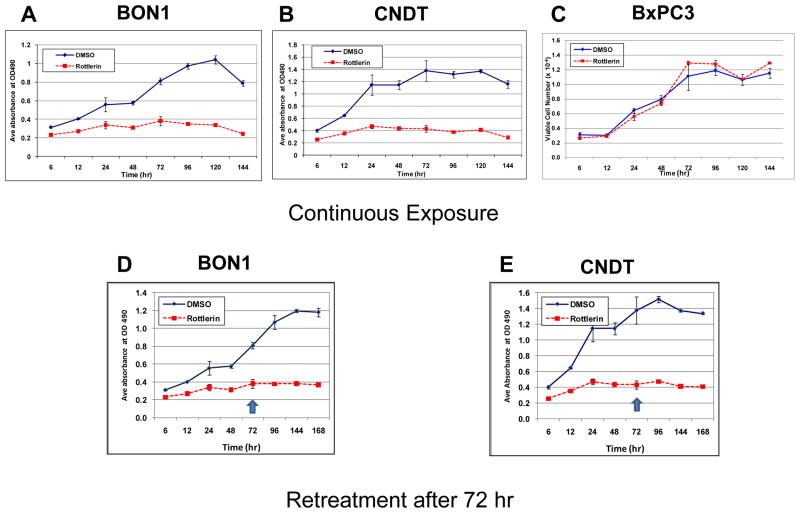
Whether neuroendocrine tumor cell lines could escape from the anti-tumor actions of PKC inhibitors was explored by long-term exposure to the inhibitors, in two experimental designs. In the first, cells were plated at a lower density to allow monitoring over longer periods for potential growth. In these “continuous” treatment studies, a PKCδ inhibitor was added at a “suboptimal” concentration, and effects on proliferation were observed as far as 144 hr after exposure (Fig. 7A&B). The decrease observed in the MTS signal from the control (vehicle-treated) cells at 144 hr represented both overgrowth of these cultures and exhaustion of the culture media. In contrast, exposure of the human cell line BxPC3, which has wild-type Ras alleles, to the same PKCδ inhibitor did not affect its growth relative to vehicle alone (Fig. 7C). To allow evaluation over even longer periods of exposure, other cultures were re-fed with fresh growth medium containing the same PKCδ inhibitor at the same concentration. In these studies, growth-inhibitory effects persisted to 168 hr of cumulative exposure (Fig. 7D&E).
Figure 7. Effects of longer term exposure of human neuroendocrine tumor cell lines to PKCδ-inhibitors.
BON1 cells (A and D) or CNDT cells (B and E) or BxPC3 cells (C) were exposed to rottlerin at a sub-optimal concentration (10 μM). Cells exposed to vehicle (DMSO) alone served as controls. At the indicated time points, cell numbers were estimated by MTS assays or enumeration of viable cells. In the cultures depicted in panels A, B and C, media was not changed. In the cultures depicted in panels D and E, fresh media containing the PKCδ inhibitor was replaced after 72 hr of exposure (arrows). Error bars represent SEM. (Fall off in cell numbers in control cultures at the longest time points likely reflect overgrowth observed in the control cultures.)
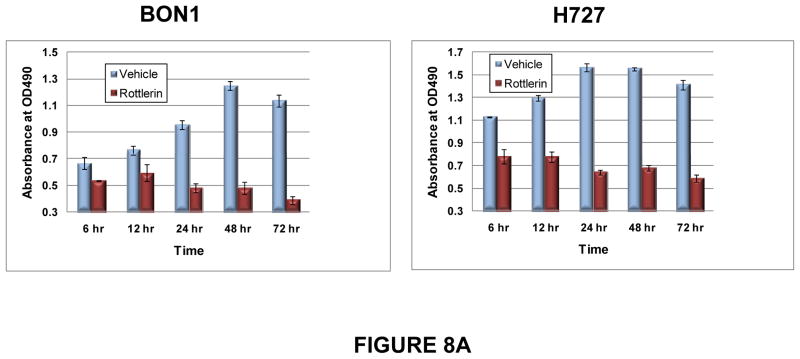
The length of exposure to PKCδ inhibition required for anti-tumor activity was next assessed. BON1 and H727 cells were exposed to a sub-optimal concentration of a PKCδ-inhibitor for different intervals of time, the inhibitor was then washed out of the culture, and the effects on cell growth were assessed over the next 72 hr. Differences in proliferation between rottlerin- and vehicle-treated cultures became statistically significant by 24 hr of exposure, and remained significant for all longer periods of exposure (Fig. 8A).
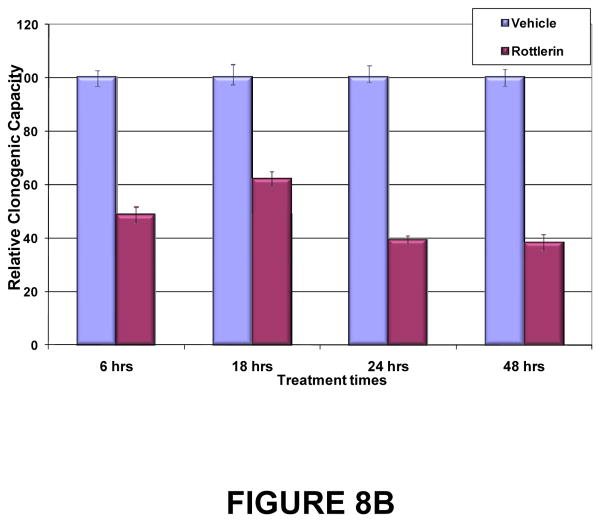
Figure 8. Duration of exposure to PKCδ inhibitors needed to inhibit tumor cell proliferation and clonogenic potential.
(A) BON1 and H727 cells were grown to 30% confluence and then exposed to vehicle or to rottlerin (10 μM) for 6, 12, 24, 48 or 72 hr. Media without inhibitor was replaced and cell numbers were estimated by MTS assay at 24, 48 and 72 hr. Shown here are the results at 72 hr of culture after each washout interval. Error bars represent SEM. Differences in proliferation between rottlerin- and vehicle-treated cultures became statistically significant (p < 0.01) by 24 hr of exposure, and remained significant for all longer periods of exposure. (B) Effects of PKCδ inhibitor on tumor cell clonogenic capacity. H727 cells were grown to 30% confluence and then exposed to vehicle or rottlerin (10 μM) for 6, 12, 24, 48 or 72 hr. Viable cells were enumerated and re-plated in media without inhibitor, and colony numbers were quantitated 96 hr later. Error bars represent SEM. P values for comparison of DMSO control and rottlerin effects on clonogenic capacity reached significance (p=0.0051) at 6 hr of exposure and remained significant for all subsequent exposure times.
LDH release assesses cytotoxic damage sufficient to compromise membrane integrity over a relatively short time-span. An alternative method, which assesses lethal, but not necessarily immediate, cumulative damage to the tumor cell is a clonogenic assay. In this assay, tumor cells which remain viable after exposure to the compound are tested for their ability to proliferate sufficiently over time to form colonies of tumor cells. H727 cells were exposed to vehicle or a PKCδ inhibitor at sub-optimal concentrations for varying durations. After re-plating of viable cells in media without inhibitor, colony numbers were quantitated over time. Significant effects of the PKCδ-inhibitors on reducing clonogenic capacity of H727 cells reached significance after as little as 6 hr of exposure, and remained significant for all subsequent exposure times (Fig. 8B). In parallel experiments, BON1 cells showed a similar drop-off in clonogenic capacity, reaching significance between 12 and 24 hr of exposure to PKCδ inhibitors (not shown).
Discussion
Ras mutations can be found in human malignancies with an overall frequency of 20%. A particularly high incidence of Ras gene mutations has been reported in malignant tumors of the pancreas (80–90%, K-Ras), in colorectal carcinomas (30–60%, K-Ras), in non-melanoma skin cancer (30–50%, H-Ras), and in hematopoietic neoplasias of myeloid origin (18–30%, K- and N-Ras) (Downward 2003; Serrano et al. 1997). In the course of studying signaling by p21Ras, we discovered discrete anti-proliferative effects of p21Ras (Rake et al. 1991; Faller et al. 1994; Chen et al. 1996). One of these properties is the activation of apoptotic signaling, resulting in rapid cell death, unless balanced by a simultaneous and independent activation of survival pathways (Chen & Faller 1995; Chen & Faller 1996; Chen & Faller 1999; Chen et al. 2003; Chen et al. 2001; Chen et al. 1998a; Chen et al. 1998b; Liou et al. 2004; Liou et al. 2000; Ma et al. 2002). This Ras-generated apoptotic signaling specifically requires PKCδ activity (Xia et al. 2009; Xia et al. 2007). In contrast, PKCδ is not generally required for development or survival of normal tissues.
Although we first discovered these anti-proliferative activities of p21Ras as properties of activated, oncogenic Ras, we have more recently shown that supra-physiological activation of endogenous c-Ras, or activation of certain Ras downstream effector pathways, will also sensitize cells to Ras-mediated apoptosis. Specifically, aberrant signaling upstream of Ras (e.g., via mutated growth factor receptors), or aberrant activation of Ras downstream pathways (PI3K, or Raf-MEK), is sufficient to sensitize cells to apoptosis when PKCδ is suppressed (Xia et al. 2007; Xia et al. 2009).
Carcinoid and other neuroendocrine tumors of the bronchopulmonary/gastrointestinal tract share a number of the same genetic abnormalities (deletions and mutations) as adenocarcinomas (Leotlela et al. 2003; Arber et al. 1997; Zikusoka et al. 2005). These abnormalities include activation of Ras directly by mutations, indirectly by loss of Ras-regulatory proteins such as NF-1, or via constitutive activation of growth factor receptors upstream of Ras or downstream effector pathways of Ras, such as PI3K and Raf/MAP kinase. Activation of (wild-type) H-Ras and Ki-Ras are detected in a significant fraction of carcinoid and other gastrointestinal neuroendocrine tumors (65% and 10%, respectively) (Liedke et al. 1998; Maitra et al. 2000). Ras can be activated in neuroendocrine tumors by either point-mutation (infrequently), constitutive signaling from upstream receptor tyrosine kinases, or loss of regulators of Ras, such as RassF1A or NF-1 (Liu et al. 2005; Stancu et al. 2003; Bausch et al. 2007). The Her-2/Neu tyrosine kinase receptor, which lies upstream of Ras, is amplified in up to 40% of gastric carcinoids, and may identify more aggressive tumor types (Evers et al. 1992). The Raf/mitogen-activated protein kinase (Raf/MAP kinase) is found to be aberrantly activated in a fraction of neuroendocrine tumors. Activating mutations of B-Raf itself are found in some neuroendocrine tumors, but infrequently in carcinoid tumors (Perren et al. 2004; Tannapfel et al. 2005; Kunnimalaiyaan & Chen 2006; Karhoff et al. 2007). In those cases where activating point-mutations of Raf are not observed, however, activation of Raf and/or the Raf-substrate MAP kinases directly downstream of Raf, is common (Tannapfel et al. 2005; Karhoff et al. 2007). This activation of the Raf/MAP kinase pathway may have a causative role in the development of neuroendocrine tumors, independent of point-mutations in B-Raf or Ras (Tannapfel et al. 2005). The PI3K pathway can be activated in neuroendocrine tumors by deletion of the tumor suppressor gene PTEN (phosphatase and tensin homologue). Loss of PTEN in neuroendocrine tumors increases in frequency with the loss of differentiation in the tumor (Wang et al. 2005), and loss of PTEN expression may represent an important step in the progression of neuroendocrine tumors (Wang et al. 2002). Cyclin D1 up-regulation in neuroendocrine tumors is quite common, likely as a result of Ras/Raf/MAP kinase pathway activation (Guo et al. 2003; Chung et al. 2000; Kawahara et al. 2002). Similarly, frequent coincident activation of the Ras effectors p38/mitogen-activated protein kinase and AKT/protein kinase B together have been reported (Guo et al. 2003; Chung et al. 2000; Kawahara et al. 2002).
Thus, as in many other human tumors, activation of Ras and Ras signaling pathways likely contribute to tumor growth and progression in many neuroendocrine tumors. However, the activation of these pathways also makes these tumors dependent upon Ras-related survival pathways, which require PKCδ for function. In the absence of this survival pathway, the proliferative properties of Ras signaling are re-directed towards apoptosis (Xia et al. 2007; Xia et al. 2009). We have shown in previous work that inhibition of PKCδ protein or activity in non-transformed cells of multiple species by genetic knockdown, dominant-negative mutants, or small-molecule chemical inhibitors, does not affect their growth or clonogenic properties, suggesting that, by its selective toxicity towards aberrant Ras signaling, this approach is tumor-targeted. Each of the three neuroendocrine tumor cell lines studied here had evidence for a different profile of Ras pathway activation, with elevated activity of p21Ras itself (as assessed by increases in GTP-bound p21Ras levels) and its downstream effector pathways in the H727 cells, activation of the Raf-MAPK pathway (as assessed by increases in phospho-ERK levels) in the CNDT cells, and some relative increases in PI3K signaling (as assessed by increases in phospho-AKT levels) in all three cell lines. Such heterogeneity in patterns of Ras pathway activation is common in most tumors, and each of these patterns of aberrant Ras signaling is sufficient to make tumor cells susceptible to apoptosis following PKCδ down-regulation (Xia et al. 2007).
We have shown in these studies that neuroendocrine tumor cell lines are susceptible to growth inhibition and apoptosis when PKCδ is down-regulated by specific genetic modes (shRNA), or by less-specific, but potentially more clinically-applicable, small molecule inhibitors. Some of these small molecule inhibitors have shown acceptable toxicity profiles in rodents (Zhang et al. 2007; Brown et al. 2005; Fryer et al. 2002, and DVF, et al., in preparation). Wash-out studies suggest a duration of exposure to PKCδ inhibitors of no more than 24 hr is required to generate a significant effect on subsequent tumor cell proliferation. More importantly, significant reductions in tumor cell clonogenic capacity in two neuroendocrine cell lines were generated by exposure to a small molecule inhibitor for as little as 6 hr.
Rottlerin was identified as a protein kinase inhibitor which inhibited PKCδ more potently than classic PKC isozymes, such as α and β (Gschwendt et al. 1994; Keenan et al. 1997; Frasch et al. 2000). We have confirmed the greater inhibitory activity of rottlerin for PKCδ relative to PKCα using PKC proteins purified from mammalian cells, in prior work (Xia et al. 2007), as well as using recombinant PKC proteins in the current report. As inhibition of PKCα is generally cytotoxic to all mammalian cells, their relative selectivity for PKCδ may contribute to the lack of toxicity of rottlerin and related compounds on normal cells. To begin development of novel PKCδ inhibitors, we carried out docking studies to predict how rottlerin binds to PKCδ. Rottlerin was docked into the catalytic binding site of several different PKC crystal structures. In many kinase/inhibitor complexes, the kinase active site is flexible; accordingly, regions known to be flexible were allowed to be free during the docking procedures. Chimeric molecules were designed using the PKCδ model developed from the rottlerin docking studies. The strategy was to retain most of the “bottom” part of Rottlerin, which was assumed to give rottlerin its specificity, but to vary the “head group,” which was assumed to bind to the hinge region of the kinase active site. A novel PKCδ inhibitor, KAM1, which is a chimeric molecule possessing portions of rottlerin and staurosporine (a non-selective pan-PKC inhibitor), was synthesized. This novel chimeric molecule demonstrated some PKCδ/PKCα inhibitory selectivity, and accordingly produced cytotoxic effects on neuroendocrine tumor cells. SAR studies of this molecule are ongoing, with the goal of developing even more selective and potent PKCδ inhibitors as potential therapeutics for carcinoid tumors.
Gastrointestinal and pulmonary carcinoid tumors are uncommon, but unfortunately are generally refractory to conventional cytotoxic chemotherapeutic and radiotherapeutic approaches. A targeted therapeutic approach, such as induction of Ras-mediated apoptosis by PKCδ inhibition, which selectively takes advantage of the very oncogenic mutations which contribute to the malignancy of the tumor, may have potential as a novel and selective therapeutic modality for these malignancies.
Acknowledgments
Funding
Supported by the Raymond and Beverly Sackler Fund for the Arts and Sciences, National Cancer Institute grants CA112102 and CA141908, Department of Defense grant PC100093, and the Karin Grunebaum Foundation for Cancer Research.
The authors gratefully acknowledge the support of Dr. Evan Vosburgh, who provided valuable advice and reagents in this work. We also thank Dr. Shuhua Xia for his contributions.
Footnotes
Declarations of interest
DVF is an inventor on patent applications relating to this work. The authors declare that they have no other competing interests.
Author contributions
DVF, ZC and LWF designed the studies and evaluated the results. ZC, LWF and AT performed experiments; RB performed molecular modeling. KAM and RWM designed and synthesized compounds. DVF, ZC and LWF wrote the manuscript. All authors read and approved the final manuscript.
Reference List
- Arber N, Neugut AI, Weinstein IB, Holt P. Molecular genetics of small bowel cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:745–748. [PubMed] [Google Scholar]
- Arnold R, Simon B, Wied M. Treatment of neuroendocrine GEP tumours with somatostatin analogues: a review. Digestion. 2000;62(Suppl 1):84–91. doi: 10.1159/000051861. [DOI] [PubMed] [Google Scholar]
- Arnold R, Wied M, Behr TH. Somatostatin analogues in the treatment of endocrine tumors of the gastrointestinal tract. Expert Opin Pharmacother. 2002;3:643–656. doi: 10.1517/14656566.3.6.643. [DOI] [PubMed] [Google Scholar]
- Bausch B, Borozdin W, Mautner VF, Hoffmann MM, Boehm D, Robledo M, Cascon A, Harenberg T, Schiavi F, Pawlu C, et al. Germline NF1 mutational spectra and loss-of-heterozygosity analyses in patients with pheochromocytoma and neurofibromatosis type 1. J Clin Endocrinol Metab. 2007;92:2784–2792. doi: 10.1210/jc.2006-2833. [DOI] [PubMed] [Google Scholar]
- Brown JM, Schwanke CM, Pershouse MA, Pfau JC, Holian A. Effects of rottlerin on silica-exacerbated systemic autoimmune disease in New Zealand mixed mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L990–L998. doi: 10.1152/ajplung.00078.2005. [DOI] [PubMed] [Google Scholar]
- Chen CY, Faller DV. Direction of p21(ras)-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene. 1995;11:1487–1498. [PubMed] [Google Scholar]
- Chen CY, Faller DV. Phosphorylation of Bcl-2 protein and association with p21(Ras) in Ras-induced apoptosis. J Biol Chem. 1996;271:2376–2379. doi: 10.1074/jbc.271.5.2376. [DOI] [PubMed] [Google Scholar]
- Chen CY, Faller DV. Selective inhibition of protein kinase C isozymes by Fas ligation. J Biol Chem. 1999;274:15320–15328. doi: 10.1074/jbc.274.22.15320. [DOI] [PubMed] [Google Scholar]
- Chen CY, Forman LW, Faller DV. Calcium-dependent immediate-early gene induction in lymphocytes is negatively regulated by p21(Ha-ras) Mol Cell Biol. 1996;16:6582–6592. doi: 10.1128/mcb.16.11.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Huang XL, Wyant H, Faller DV. Acute and chronic effects of p21Ras activation on apoptosis and DNA stability. 2003. submitted for publication. [Google Scholar]
- Chen CY, Juo P, Liou J, Yu Q, Blenis J, Faller DV. Activation of FADD and Caspase 8 in Ras-mediated apoptosis. Cell Growth Differ. 2001;12:297–306. [PubMed] [Google Scholar]
- Chen CY, Liou J, Forman LW, Faller DV. Correlation of genetic instability and apoptosis in the presence of oncogenic Ki-Ras. Cell Death Differentiation. 1998a;5:984–995. doi: 10.1038/sj.cdd.4400448. [DOI] [PubMed] [Google Scholar]
- Chen CY, Liou J, Forman LW, Faller DV. Differential regulation of discrete apoptotic pathways by Ras. J Biol Chem. 1998b;273:16700–16709. doi: 10.1074/jbc.273.27.16700. [DOI] [PubMed] [Google Scholar]
- Chung DC, Brown SB, Graeme-Cook F, Seto M, Warshaw AL, Jensen RT, Arnold A. Overexpression of cyclin D1 occurs frequently in human pancreatic endocrine tumors. J Clin Endocrinol Metab. 2000;85:4373–4378. doi: 10.1210/jcem.85.11.6937. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Evers BM, Rady PL, Tyring SK, Sanchez RL, Rajaraman S, Townsend CM, Jr, Thompson JC. Amplification of the HER-2/neu protooncogene in human endocrine tumors. Surgery. 1992;112:211–217. [PubMed] [Google Scholar]
- Faller DV, Mundschau LJ, Forman LW, Quinones MA. v-mos suppresses platelet-derived growth factor (PDGF) type-β receptor autophosphorylation and inhibits PDGF-BB-mediated signal transduction. J Biol Chem. 1994;269:5022–5029. [PubMed] [Google Scholar]
- Frank M, Klose KJ, Wied M, Ishaque N, Schade-Brittinger C, Arnold R. Combination therapy with octreotide and alpha-interferon: effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol. 1999;94:1381–1387. doi: 10.1111/j.1572-0241.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- Frasch SC, Henson PM, Kailey JM, Richter DA, Janes MS, Fadok VA, Bratton DL. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cdelta. J Biol Chem. 2000;275:23065–23073. doi: 10.1074/jbc.M003116200. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Hsu AK, Wang Y, Henry M, Eells J, Gross GJ. PKC-delta inhibition does not block preconditioning-induced preservation in mitochondrial ATP synthesis and infarct size reduction in rats. Basic Res Cardiol. 2002;97:47–54. doi: 10.1007/s395-002-8387-x. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Guda K, Natale L, Markowitz SD. An improved method for staining cell colonies in clonogenic assays. Cytotechnology. 2007;54:85–88. doi: 10.1007/s10616-007-9083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Wu X, Shimoide AT, Wong J, Moatamed F, Sawicki MP. Frequent overexpression of cyclin D1 in sporadic pancreatic endocrine tumours. J Endocrinol. 2003;179:73–79. doi: 10.1677/joe.0.1790073. [DOI] [PubMed] [Google Scholar]
- Karhoff D, Sauer S, Schrader J, Arnold R, Fendrich V, Bartsch DK, Horsch D. Rap1/B-Raf signaling is activated in neuroendocrine tumors of the digestive tract and Raf kinase inhibition constitutes a putative therapeutic target. Neuroendocrinology. 2007;85:45–53. doi: 10.1159/000100508. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kammori M, Kanauchi H, Noguchi C, Kuramoto S, Kaminishi M, Endo H, Takubo K. Immunohistochemical prognostic indicators of gastrointestinal carcinoid tumours. Eur J Surg Oncol. 2002;28:140–146. doi: 10.1053/ejso.2001.1229. [DOI] [PubMed] [Google Scholar]
- Keenan C, Goode N, Pears C. Isoform specificity of activators and inhibitors of protein kinase C gamma and delta. FEBS Lett. 1997;415:101–108. doi: 10.1016/s0014-5793(97)01104-6. [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase C delta (PKC delta): activation mechanisms and functions. J Biochem. 2002;132:831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- Kunnimalaiyaan M, Chen H. The Raf-1 pathway: a molecular target for treatment of select neuroendocrine tumors? Anticancer Drugs. 2006;17:139–142. doi: 10.1097/00001813-200602000-00004. [DOI] [PubMed] [Google Scholar]
- Leotlela PD, Jauch A, Holtgreve-Grez H, Thakker RV. Genetics of neuroendocrine and carcinoid tumours. Endocr Relat Cancer. 2003;10:437–450. doi: 10.1677/erc.0.0100437. [DOI] [PubMed] [Google Scholar]
- Li GZ, Eller MS, Hanna K, Gilchrest BA. Signaling pathway requirements for induction of senescence by telomere homolog oligonucleotides. Exp Cell Res. 2004;301:189–200. doi: 10.1016/j.yexcr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Liedke M, Karnbach C, Kalinin V, Herbst B, Frilling A, Broelsch CE. Detection of H-ras and K-ras in tumors of gastrointestinal-pancreatic system. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:255–259. [PubMed] [Google Scholar]
- Liou JS, Chen CY, Chen JS, Faller DV. Oncogenic Ras mediates apoptosis in response to protein kinase C inhibition through the generation of reactive oxygen species. J Biol Chem. 2000;275:39001–39011. doi: 10.1074/jbc.M007154200. [DOI] [PubMed] [Google Scholar]
- Liou JS, Chen J-C, Faller DV. Characterization of p21Ras-mediated apoptosis induced by Protein Kinase C inhibition and application to human tumor cell lines. J Cell Physiol. 2004;198:277–294. doi: 10.1002/jcp.10409. [DOI] [PubMed] [Google Scholar]
- Liu L, Broaddus RR, Yao JC, Xie S, White JA, Wu TT, Hamilton SR, Rashid A. Epigenetic alterations in neuroendocrine tumors: methylation of RAS-association domain family 1, isoform A and p16 genes are associated with metastasis. Mod Pathol. 2005;18:1632–1640. doi: 10.1038/modpathol.3800490. [DOI] [PubMed] [Google Scholar]
- Ma P, Magut M, Chen X, Chen CY. P53 is necessary for the apoptotic response mediated by a transient increase of Ras activity. Mol Cell Biol. 2002;22:2928–2938. doi: 10.1128/MCB.22.9.2928-2938.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Krueger JE, Tascilar M, Offerhaus GJ, Angeles-Angeles A, Klimstra DS, Hruban RH, Albores-Saavedra J. Carcinoid tumors of the extrahepatic bile ducts: a study of seven cases. Am J Surg Pathol. 2000;24:1501–1510. doi: 10.1097/00000478-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Oberg K. Neuroendocrine gastrointestinal tumors--a condensed overview of diagnosis and treatment. Ann Oncol. 1999;10(Suppl 2):S3–S8. doi: 10.1093/annonc/10.suppl_2.s3. [DOI] [PubMed] [Google Scholar]
- Oberg K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S111–S114. doi: 10.1093/annonc/12.suppl_2.s111. [DOI] [PubMed] [Google Scholar]
- Parekh D, Ishizuka J, Townsend CM, Jr, Haber B, Beauchamp RD, Karp G, Kim SW, Rajaraman S, Greeley G, Jr, Thompson JC. Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas. 1994;9:83–90. doi: 10.1097/00006676-199401000-00013. [DOI] [PubMed] [Google Scholar]
- Perren A, Schmid S, Locher T, Saremaslani P, Bonvin C, Heitz PU, Komminoth P. BRAF and endocrine tumors: mutations are frequent in papillary thyroid carcinomas, rare in endocrine tumors of the gastrointestinal tract and not detected in other endocrine tumors. Endocr Relat Cancer. 2004;11:855–860. doi: 10.1677/erc.1.00841. [DOI] [PubMed] [Google Scholar]
- Rake JB, Quinones MA, Faller DV. Inhibition of platelet-derived growth factor-mediated signal transduction by transforming ras. Suppression of receptor autophosphorylation. J Biol Chem. 1991;266:5348–5352. [PubMed] [Google Scholar]
- Schuller HM, Falzon M, Gazdar AF, Hegedus T. Cell type-specific differences in metabolic activation of N-nitrosodiethylamine by human lung cancer cell lines. IARC Sci Publ. 1987:138–140. [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16(INK4a) Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Stancu M, Wu TT, Wallace C, Houlihan PS, Hamilton SR, Rashid A. Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol. 2003;16:1189–1198. doi: 10.1097/01.MP.0000097362.10330.B1. [DOI] [PubMed] [Google Scholar]
- Tannapfel A, Vomschloss S, Karhoff D, Markwarth A, Hengge UR, Wittekind C, Arnold R, Horsch D. BRAF gene mutations are rare events in gastroenteropancreatic neuroendocrine tumors. Am J Clin Pathol. 2005;123:256–260. [PubMed] [Google Scholar]
- Van Buren G, Rashid A, Yang AD, Abdalla EK, Gray MJ, Liu W, Somcio R, Fan F, Camp ER, Yao JC, et al. The development and characterization of a human midgut carcinoid cell line. Clin Cancer Res. 2007;13:4704–4712. doi: 10.1158/1078-0432.CCR-06-2723. [DOI] [PubMed] [Google Scholar]
- Wang GG, Yao JC, Worah S, White JA, Luna R, Wu TT, Hamilton SR, Rashid A. Comparison of genetic alterations in neuroendocrine tumors: frequent loss of chromosome 18 in ileal carcinoid tumors. Mod Pathol. 2005;18:1079–1087. doi: 10.1038/modpathol.3800389. [DOI] [PubMed] [Google Scholar]
- Wang L, Ignat A, Axiotis CA. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:139–146. doi: 10.1097/00129039-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Xia S, Chen Z, Forman LW, Faller DV. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal. 2009;21:502–508. doi: 10.1016/j.cellsig.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Forman LW, Faller DV. Protein Kinase C{delta} is required for survival of cells expressing activated p21RAS. J Biol Chem. 2007;282:13199–13210. doi: 10.1074/jbc.M610225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson’s disease. J Pharmacol Exp Ther. 2007;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]
- Zikusoka MN, Kidd M, Eick G, Latich I, Modlin IM. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer. 2005;104:2292–2309. doi: 10.1002/cncr.21451. [DOI] [PubMed] [Google Scholar]