Figure 6.
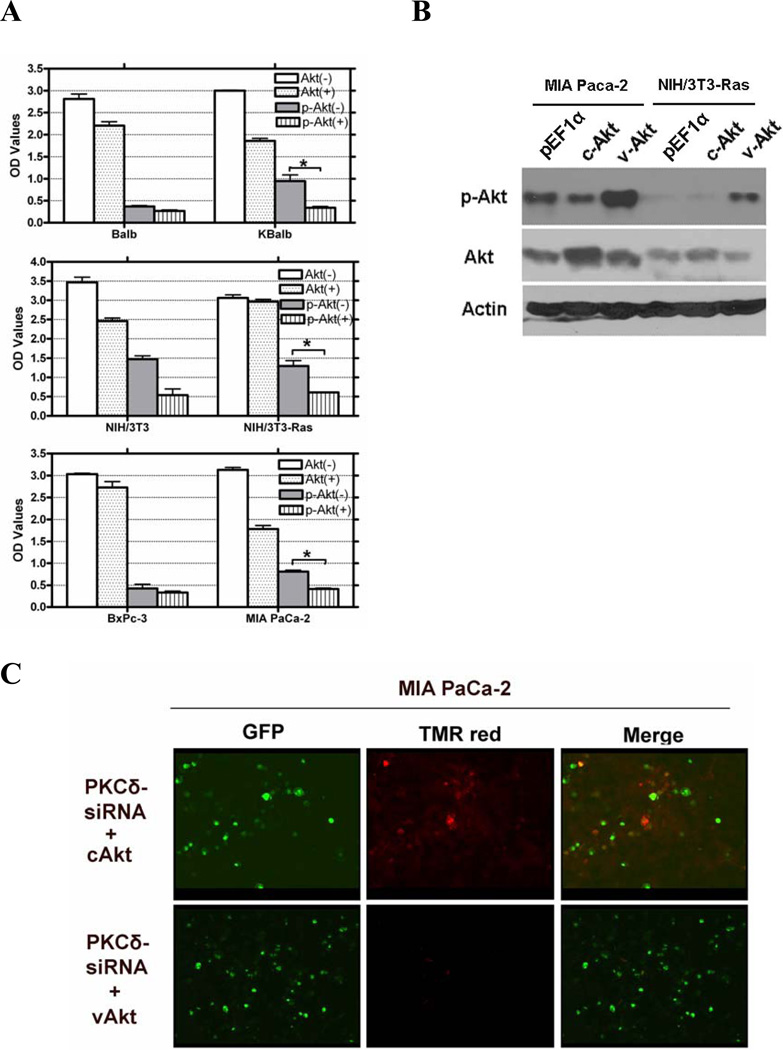
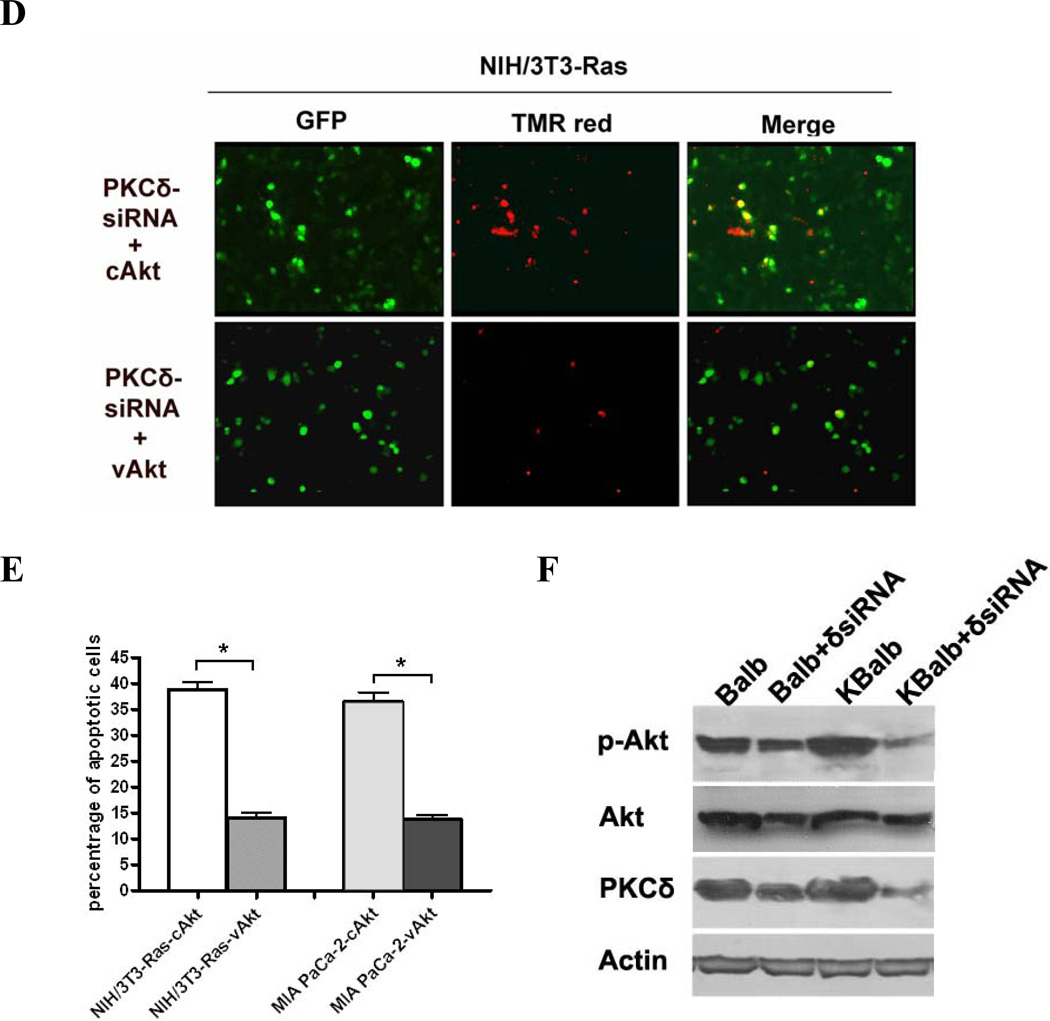
Regulation of AKT activity by PKCδ. (A) Treatment with rottlerin reduces Akt activity in cells expressing activated Ras. All cells were treated with 20 µM rottlerin for 60 h in 96-well plates. Total Akt and p-Akt levels were assayed using a SuperArray Case ELISA kit (*, p<0.005). (+): treated with 20 µM rottlerin; (−): treated with vehicle (control); (B) pAkt protein levels in MIA PaCa-2 and NIH/3T3-Ras cells, assayed by immunoblotting with an antibody specific for phosphorylated serine473 Akt, or for total Akt, or for β-actin. Both cell lines were transfected with pEGFP and with pEF1α empty vector, or pEF1α-cAKT, or pEF1α-vAkt. GFP expression was used to normalize for transfection efficiency. (C & D) Activated Akt rescues cells from apoptosis induced by PKCδ knock-down. MIA PaCa-2 and NIH/3T3-Ras cells were cultured on 4-well chamber slides and were co-transfected with pRNA-U6.1-GFP-PKCδ hairpin vector and with pEF1α-vAkt or pEF1α-cAkt vectors. The PKCδ-hairpin vector efficiently suppressed PKCδ levels in these experiments by at least 85% (e.g., see Fig. 3A). After 72 h, cells were fixed with 4% paraformaldehyde for 1 h at room temperature, and then subjected to TUNEL assay. Shown are 200x magnifications. (E) Quantitation of apoptotic cells in the transfected populations (*, p < 0.001). (F) Immunoblot of lysates from Balb or KBalb cells treated with PKCδ-specific siRNA or scrambled, control siRNA. Antibodies against PKCδ, total Akt, phospho-serine473 Akt, and β-actin were used. The results are representative of at least two independent experiments.