Abstract
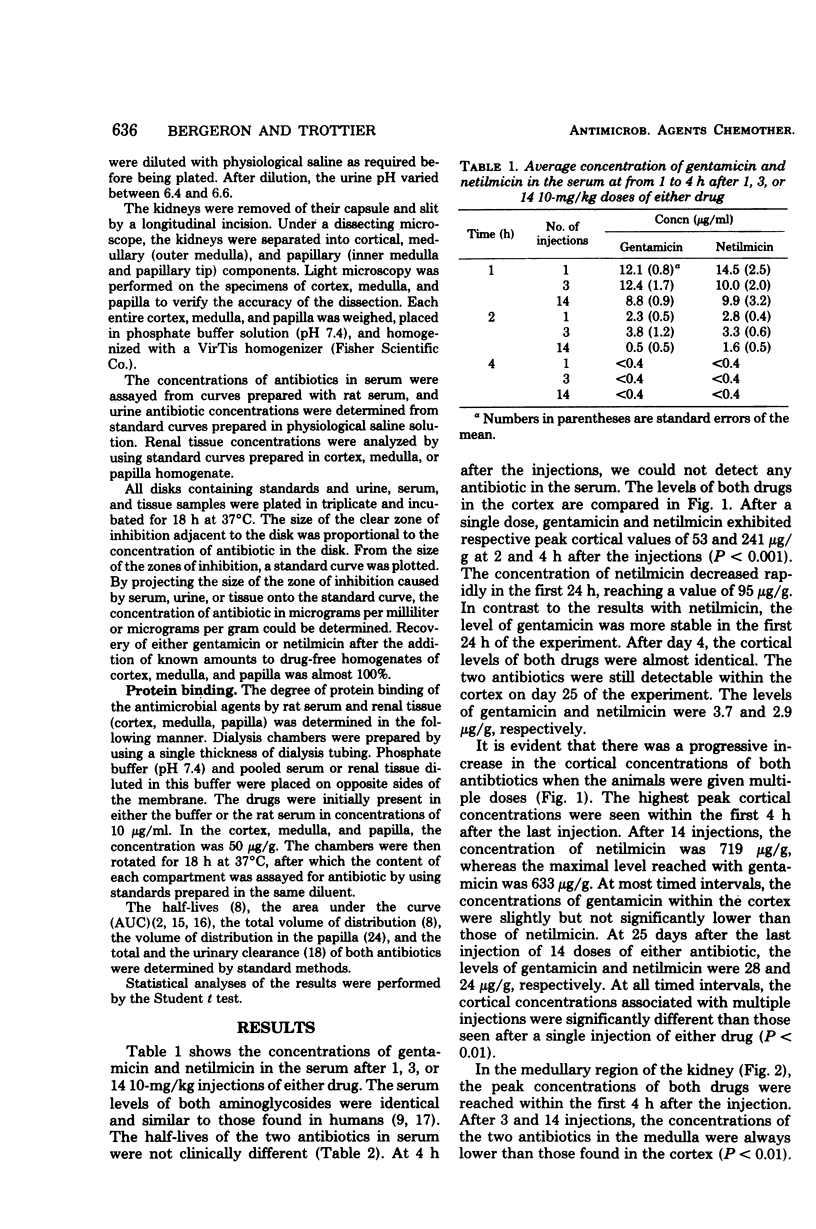
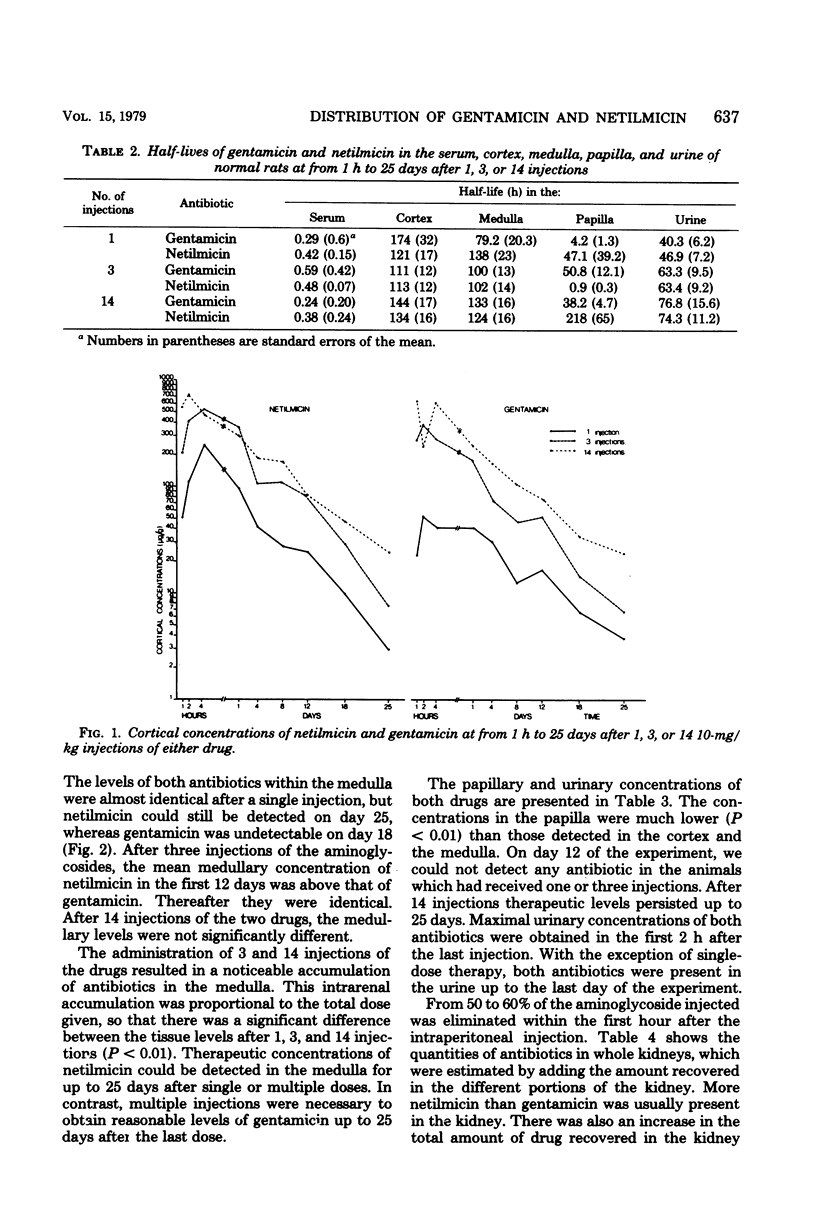
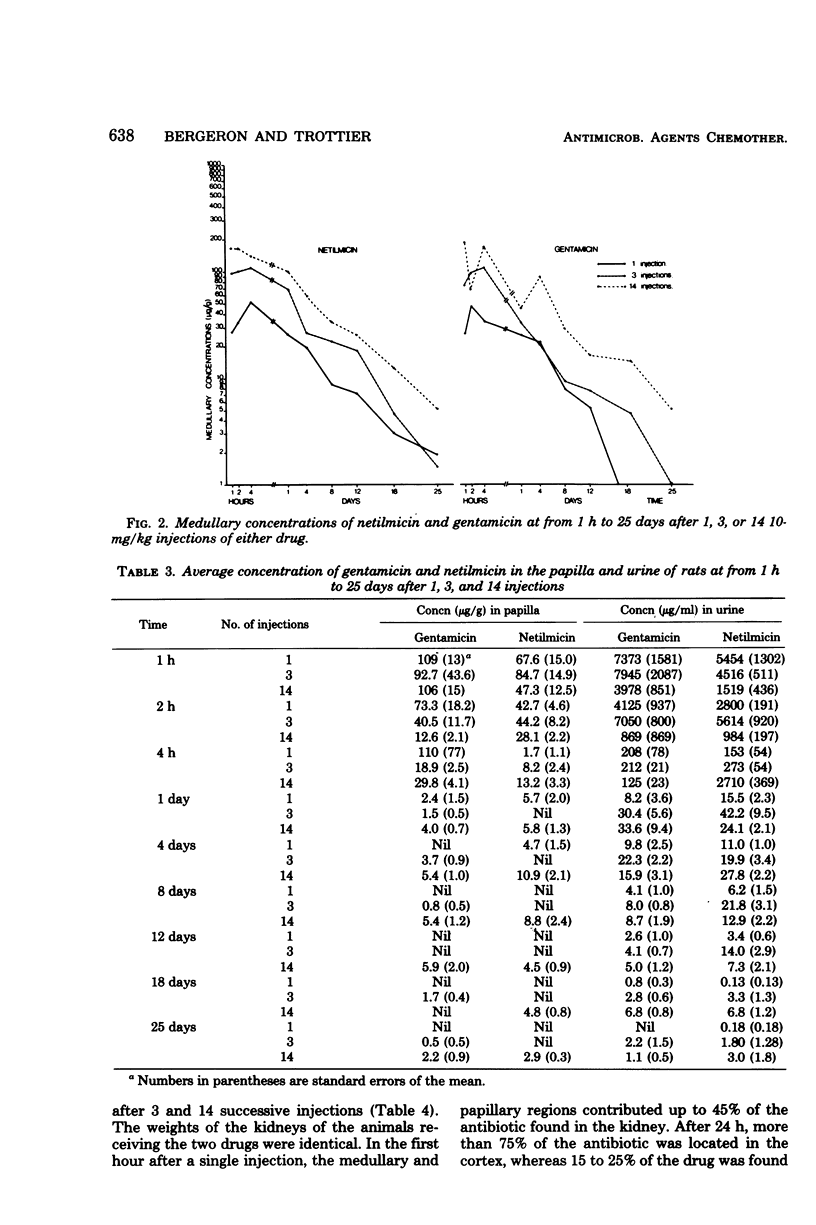
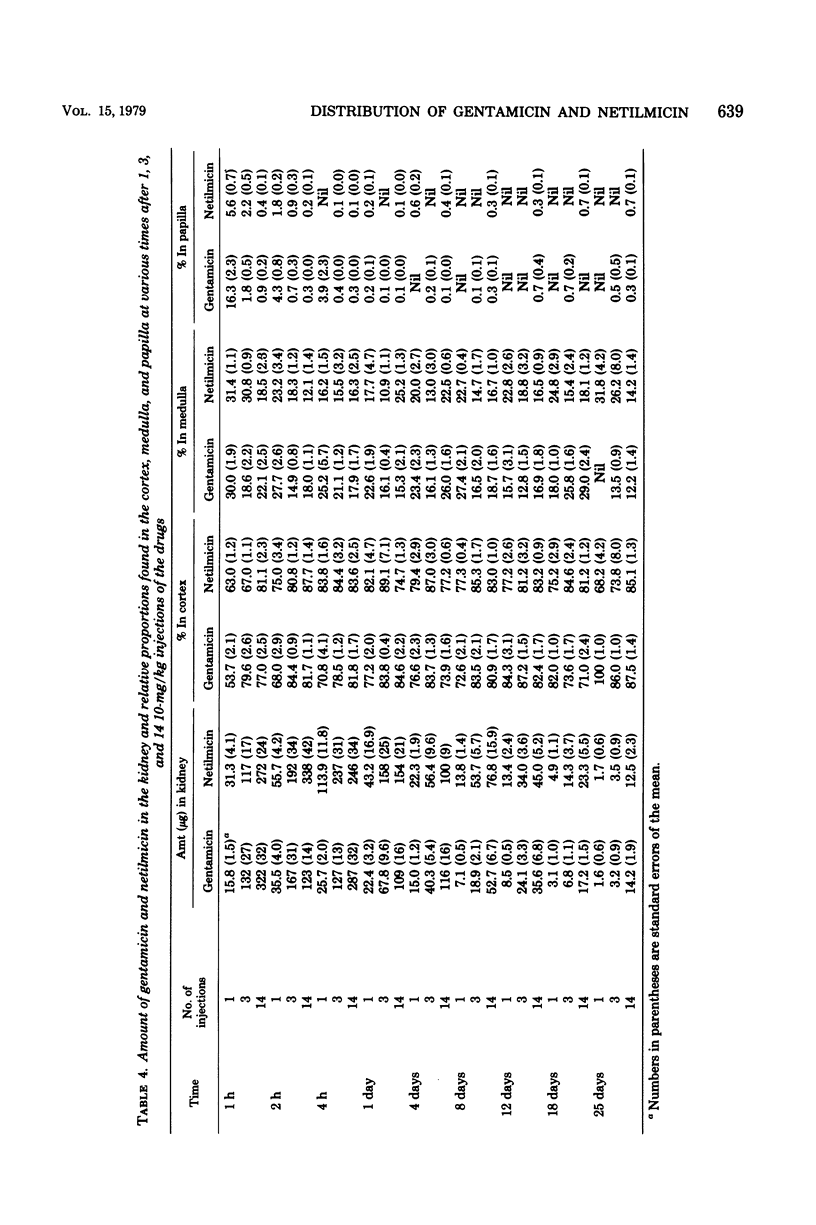
In this study, the comparative intrarenal distribution and accumulation of both gentamicin and netilmicin were investigated in normal rats. The animals received 1, 3, or 14 injections of 10 mg of gentamicin or netilmicin per kg. A total of 324 animals and 648 kidneys were analyzed. These animals were sacrificed at from 1 h to 25 days after the intraperitoneal injections. At each timed interval, the serum, urine, cortex, medulla, and papilla were analyzed for antimicrobial content. The peak serum values of gentamicin (8 to 12 μg/ml) and netilmicin (9 to 14 μg/ml) were close to those found in humans. There was a progressive increase in the cortical concentrations of both antibiotics from a low of 53 μg/g to a high of 719 μg/g when the animals were given increasing doses of the agents. At most timed intervals, the concentrations of gentamicin within the cortex were slightly lower than those of netilmicin. The accumulation of the drugs was also demonstrated in the medulla, where therapeutic levels could be detected for up to 25 days after the cessation of therapy. Even though gentamicin and netilmicin were transiently present in the papilla after 1 or 3 injections, both drugs could still be detected in the papilla on day 25 after 14 doses. The urinary concentration closely paralleled the papillary concentration. If applicable to humans, the persistence of high levels of both drugs within the medulla and papilla may have therapeutic implications.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barza M., Pinn V., Tanguay P., Murray T. Nephrotoxicity of newer cephalosporins and aminoglycosides alone and in combination in a rat model. J Antimicrob Chemother. 1978 May;4 (Suppl A):59–68. doi: 10.1093/jac/4.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- Bergan T., Brodwall E. K. Human pharmacokinetics of a sulfamethoxazole-trimethoprim combination. Acta Med Scand. 1972 Dec;192(6):483–492. doi: 10.1111/j.0954-6820.1972.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Bowman R. L., Silverblatt F. J., Kaloyanides G. J. Comparison of the nephrotoxicity of netilmicin and gentamicin in rats. Antimicrob Agents Chemother. 1977 Oct;12(4):474–478. doi: 10.1128/aac.12.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P. J., Brown A., Miller G., Long J. F. Renal extraction of gentamicin in anesthetized dogs. Antimicrob Agents Chemother. 1976 Aug;10(2):277–282. doi: 10.1128/aac.10.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P. J., Miller G. H., Brown A. D., Long J. F., Waitz J. A. Renal pharmacology of netilmicin. Antimicrob Agents Chemother. 1977 May;11(5):821–825. doi: 10.1128/aac.11.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre J., Rudhardt M., Blanchard P., Regamey C. Persistence of sisomicin and gentamicin in renal cortex and medulla compared with other organs and serum of rats. Kidney Int. 1976 Dec;10(6):444–449. doi: 10.1038/ki.1976.131. [DOI] [PubMed] [Google Scholar]
- Gilbert D. N., Plamp C., Starr P., Bennet W. M., Houghton D. C., Porter G. Comparative nephrotoxicity of gentamicin and tobramycin in rats. Antimicrob Agents Chemother. 1978 Jan;13(1):34–40. doi: 10.1128/aac.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Kurtz T. W., Weller J. M. In vitro uptake of gentamicin by rat renal cortical tissue. Antimicrob Agents Chemother. 1977 Aug;12(2):192–194. doi: 10.1128/aac.12.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlmeter G., Kamme G. Letter: Prolonged excretion of gentamicin in a patient with umimpaired renal function. Lancet. 1975 Feb 1;1(7901):286–286. doi: 10.1016/s0140-6736(75)91197-6. [DOI] [PubMed] [Google Scholar]
- Kornguth M. L., Kunin C. M. Distribution of gentamicin and amikacin in rabbit tissues. Antimicrob Agents Chemother. 1977 Jun;11(6):974–977. doi: 10.1128/aac.11.6.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F. C., Kleit S. A. Renal parenchymal accumulation of aminoglycoside antibiotics in rats. J Infect Dis. 1974 Dec;130(6):656–659. doi: 10.1093/infdis/130.6.656. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Yum M. N., Kleit S. A. Comparative nephrotoxicities of netilmicin and gentamicin in rats. Antimicrob Agents Chemother. 1976 Nov;10(5):845–849. doi: 10.1128/aac.10.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand M. APL computer program for the analytical and numerical integration of hormonal disappearance data. Can J Physiol Pharmacol. 1972 Aug;50(8):845–852. doi: 10.1139/y72-122. [DOI] [PubMed] [Google Scholar]
- Normand M., Fortier C. Numerical versus analytical integration of hormonal disappearance data. Can J Physiol Pharmacol. 1970 May;48(5):274–281. doi: 10.1139/y70-046. [DOI] [PubMed] [Google Scholar]
- Panwalker A. P., Malow J. B., Zimelis V. M., Jackson G. G. Netilmicin: clinical efficacy, tolerance, and toxicity. Antimicrob Agents Chemother. 1978 Feb;13(2):170–176. doi: 10.1128/aac.13.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMEY T. A., GOVAN D. E., PALMER J. M. THE LOCALIZATION AND TREATMENT OF URINARY TRACT INFECTIONS: THE ROLE OF BACTERICIDAL URINE LEVELS AS OPPOSED TO SERUM LEVELS. Medicine (Baltimore) 1965 Jan;44:1–36. doi: 10.1097/00005792-196501000-00001. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J., Vance J. W., Cumbo T. J., Abrutyn E., DeLattre M., Gerbracht L. M. Gentamicin disposition and tissue accumulation on multiple dosing. J Pharmacokinet Biopharm. 1977 Dec;5(6):559–577. doi: 10.1007/BF01059684. [DOI] [PubMed] [Google Scholar]
- Szwed J. J., Luft F. C., Black H. R., Elliott R. A., Kleit S. A. Comparison of the distribution of tobramycin and gentamicin in body fluids of dogs. Antimicrob Agents Chemother. 1974 May;5(5):444–446. doi: 10.1128/aac.5.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton A., Carter G. G., Craig T. J., Bryant H. H., Herbst D. V., Walker W. G. Comparison of the intrarenal disposition of tobramycin and gentamicin: therapeutic and toxicologic answers. J Antimicrob Chemother. 1978 May;4 (Suppl A):13–22. doi: 10.1093/jac/4.suppl_a.13. [DOI] [PubMed] [Google Scholar]
- Whelton A., Sapir D. G., Carter G. G., Kramer J., Walker W. G. Intrarenal distribution of penicillin, cephalothin, ampicillin and oxytetracycline during varied states of hydration. J Pharmacol Exp Ther. 1971 Nov;179(2):419–428. [PubMed] [Google Scholar]
- Whelton A., Walker W. G. Editorial: Intrarenal antibiotic distribution in health and disease. Kidney Int. 1974 Sep;6(3):131–137. doi: 10.1038/ki.1974.91. [DOI] [PubMed] [Google Scholar]


