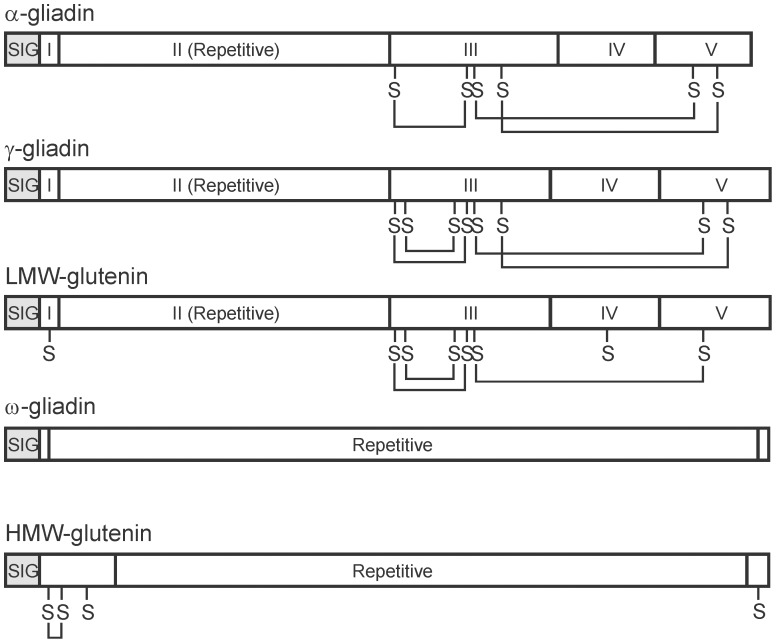
Figure 1. General Structure of the wheat prolamins.
The general structure of the wheat prolamin classes is diagrammed showing the main sequence domains, conserved cysteine residues (S), and intramolecular disulfide bonds (lines connecting Ss). The signal peptides (SIG) are shaded. The mature polypeptide sequence of the α- and γ-gliadins and LMW-glutenins are composed of five sections: (I) a short non-repetitive peptide, (II) the repetitive domain composed of variations of short motifs, (III) a non-repetitive region containing most of the cysteine residues, (IV) a glutamine-rich domain, and (V) the C-terminal non-repetitive domain containing at least one cysteine residue. The ω-gliadins usually have no cysteines and therefore no disulfide bonds. The disulfide bonds are taken from references: α-gliadins [15], γ-gliadins [16], and the HMW- and LMW-glutenins [17].