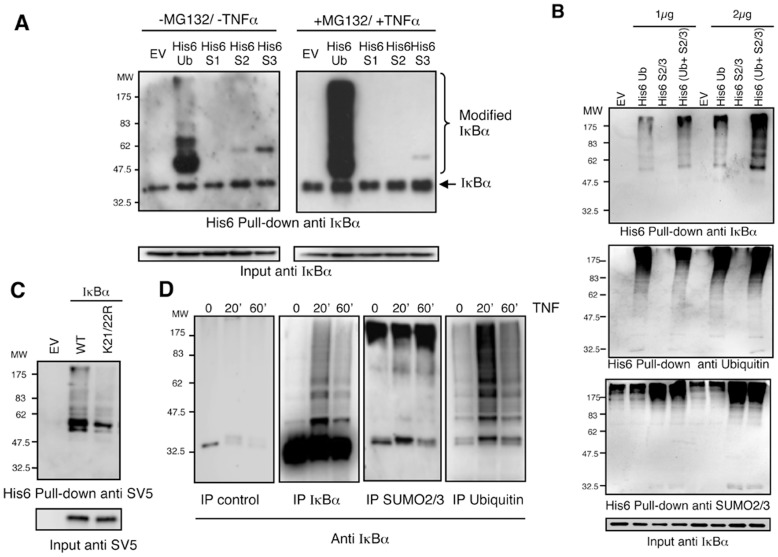
Figure 3. IκBα is modified by ubiquitin chains containing SUMO-2/3.
(A) HEK293 cells were transfected with the indicated plasmids, pre-treated or not with MG132 and stimulated or not with TNFα. His6-ubiquitylated or SUMOylated proteins were purified using denaturing conditions and Ni2+ chromatography. (B) HEK293 cells were transfected with the indicated plasmids at two different concentrations 1 µg or 2 µg of each constructs. Empty vector (EV) was also used to compensate plasmid DNA to final concentration of 2 or 4 µg respectively. Cells were pre-treated with MG132 and stimulated with TNFα during the indicated times. His6-ubiquitylated or SUMOylated proteins were purified using denaturing conditions and Ni2+ chromatography. Captured material was analysed by western-blot with the indicated antibodies. (C) HEK293 cells were transfected with IκBα-SV5 WT or mutated on K21 and K22 in the presence of His6Ubiquitin, His6-SUMO2 and His6-SUMO3, pre-treated with MG132 and stimulated with TNFα. His6-modified proteins were purified using denaturing conditions and Ni2+ chromatography procedure (D) Time-course modification of IκBα after TNFα-stimulation analysed by immunoprecipitations with anti-IgG control, anti-ubiquitin, anti-IκBα and anti-SUMO-2/3 antibodies. Cells were treated with MG132, stimulated with TNFα and lysates were submitted to immunoprecipitation experiments as indicated. Precipitated material was analysed by western-blot with anti-IκBα antibody.