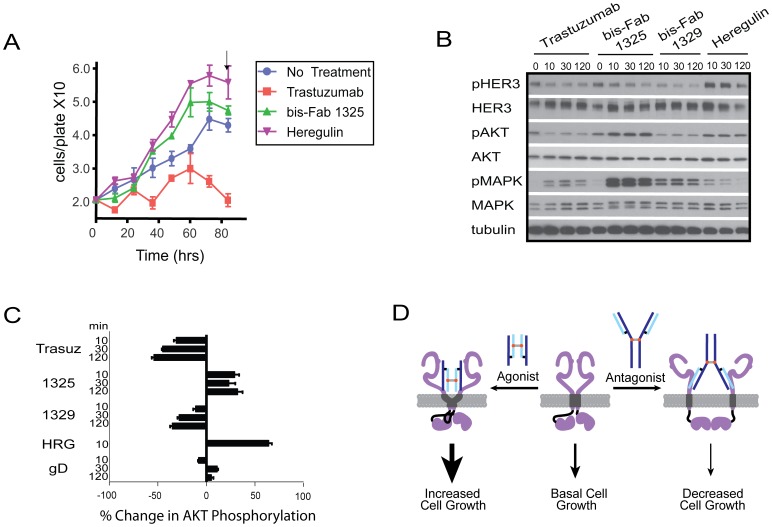
Figure 4. Analysis of bis-Fab agonist activity in BT474 cells.
(a) A time course of cell growth activity in BT474 cells in the presence of 100 nM trastuzumab, 100 nM bis-Fab 1325, or 10 nM heregulin. BT474 were cultured in media containing 10% fetal bovine serum for up to 84 hours. At 12-hour intervals total number of cells were determined; three plates from each treatment group were counted for the total number of cells and plotted as the mean cell count. The error bars indicate standard deviation from the mean. At approximately 60 hours (indicated by the arrow) the cells reached confluence in the agonist treatment groups. (b) BT474 cells were treated with 100 nM of trastuzumab, 100 nM of bis-Fab 1325, or 100 nM of bis-Fab 1329 for 10, 30 and 120 minutes. At times indicated, cell lysates were prepared and analyzed by immunoblotting using phospho-specific antibodies for HER3, AKT, and MAPK as well as antibodies recognizing total protein. Data are representative of three independent experiments. (c) Quantification of AKT phosphorylation after treatment with 100 nM bis-Fab 1325, 100 nM trastuzumab, 2 nM heregulin and a non-specific control antibody (anti-gD) by PathScan p-AKT1 (S473) ELISA. All data points were collected in triplicates and the mean of the triplicate absorbance values were used to calculate the percent change in pAKT compared to untreated control group. The error bars indicate standard deviation from the mean. Data are representative of three independent experiments. (d) A model for the HER2 dimerization patterns induced by either the agonist antibody-analogs or trastuzumab. The diagram depicts three potential dimer conformations; 1) the basal state induced by high cell surface density, 2) the activated state induced by the bis-Fab agonist, and 3) the inhibited state stabilized by trastuzumab. The cell growth activity of agonist bis-Fabs may be due to stabilization of an allosterically activated conformation between HER2–HER2 dimers. Trastuzumab's antagonistic activity may arise from dimer orientations that favor the inactive allosteric interactions between kinases. A non-stabilized dimer may represent the basal state where interactions between the juxtamembrane (black line) loop of the activator kinase and the C-terminal lobe of the receiver kinase are not fully stabilized without agonist binding.