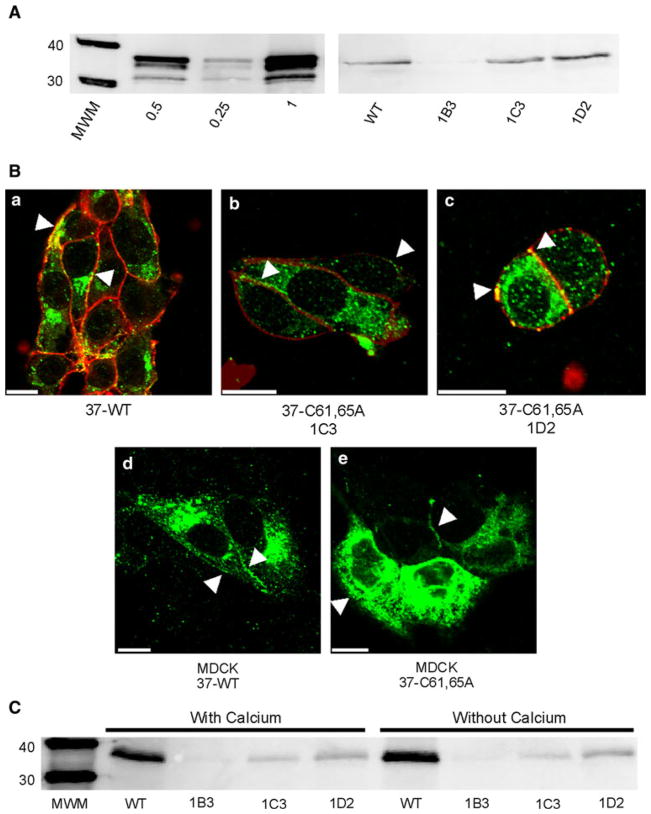
Fig. 1.
Expression and localization of Cx37-C61,65A and Cx37-WT are comparable. A Western blots of GST-37CT loaded in different amounts (left) and total protein isolated from iRin37 and three clones (1B3, 1C3 and 1D2) of iRin37-C61,65A cells (right). iRin cells were induced for 24 h with 2 μg/ml doxycycline, whole-cell protein was isolated and 50 μg of total cell protein was loaded for each sample. Expression levels for each cell line, determined by comparing band intensities of the samples to the standard curve generated using GST-37CT, were 3.7, 4.5 and 5.1 pmol/mg of total cell protein for clones 1B3, 1C3 and 1D2, respectively, and 4.5 pmol/mg protein for iRin37 cells. B Confocal images of Cx37 (green) localization with biotinylated membrane proteins (red) in induced iRin37 (37-WT) (a), iRin37-C61,65A 1C3 (37-C61,65A 1C3) (b) and iRin37-C61,65A 1D2 (37-C61,65A 1D2) (c) cells showing the presence of punctate labeling at appositional and nonappositional membranes (arrows). Confocal images of Cx37 (green) localization in MDCK cells transiently transfected to express Cx37-WT (MDCK 37-WT) (d) or Cx37-C61,65A (MDCK 37-C61,65A) (e) also showing the presence of punctate labeling at appositional and nonappositional membranes (arrows). Scale bars = 10 μm. C Western blot of membrane localized Cx37 isolated from Cx37-WT or -C61,65A expressing iRin cells. Induced iRin37 (WT) and iRin37-C61,65A clones 1B3, 1C3 and 1D2 were DTT- and maleimide-biotin-treated and harvested, and protein was immunoprecipitated with streptavidin and immunoblotted with anti-Cx37 antibody. Solutions either contained or lacked calcium. Detected Cx37 indicates membrane insertion of this protein. Differences in band intensity in part reflect different cell densities in initial samples. Loaded 25 μl of each sample