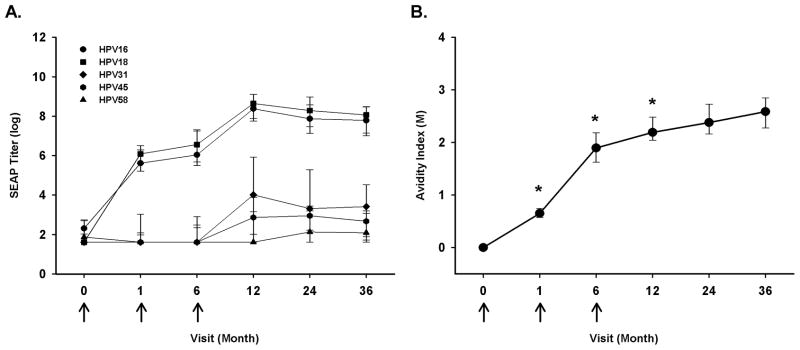
Figure 1.
Kinetic profile of neutralizing anti-HPV-16, -18, -31, -45, and -58 antibody responses and HPV-16 avidity following enrollment into HPV-16 and -18 L1 VLP vaccine trial among HPV uninfected women. Participants (n = 12) received three doses of Cervarix® at months 0, 1, and 6 (arrows). The participant’s neutralizing antibody responses (A) were evaluated in a pseudovirion-based neutralization assay from enrollment to 36 months post-enrollment. The lines represent median values at each visit, and the error bars represent the 25th to 75th percentiles. The participant’s anti-HPV-16 avidity response (B) was evaluated from enrollment to 36 months post-enrollment. The lines represent median values at each visit. Wilcoxon test was used to calculate the p-value (*-p<0.05) and represents the comparison between current visit and preceding visit.