Figure 3.
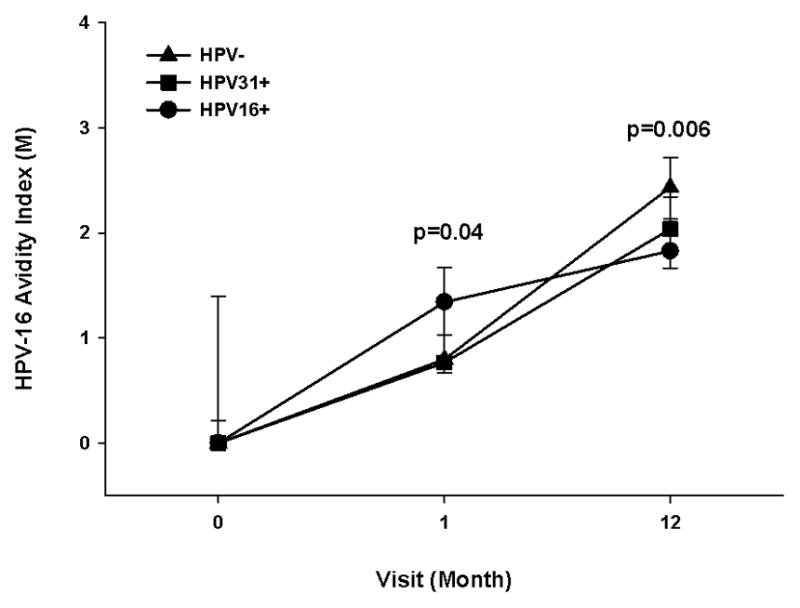
Effects of HPV-16 infection at enrollment on Cervarix® -induced anti-HPV-16 avidity response. Participants with an HPV-16 infection (HPV16+, n=18), HPV-31 infection (HPV31+, n=13), and HPV DNA negative, self-reported virgins (HPV-, n=15) at enrollment had serum tested for avidity at enrollment (month 0), months 1 and 12. The lines represent median values at each visit, and the error bars represent the 25th to 75th percentiles. The Wilcoxon test was used to calculate the p value between uninfected and infected individuals at each visit (infection based on enrollment HPV DNA assessment). A p-value <0.05 was considered significant, and the indicated p values are based on the comparison between the HPV16+ and HPV- groups at the same visit.
