Table 1.
Catalytic Dehydrogenative Diamination[a]
| Entry | Substrate (1) | Product (5) | Yield (%)[e] |
|---|---|---|---|
| 1 |
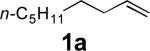
|
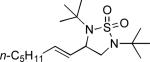
|
68 |
| 2 |
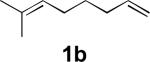
|
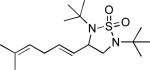
|
47 |
| 3 |
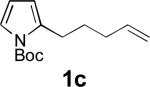
|
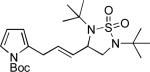
|
74 |
| 4 |
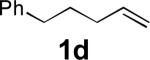
|
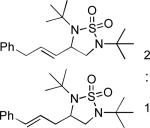
|
77 |
| 5[b] |
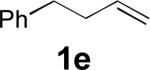
|
|
45 |
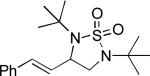
| 6 |
|
|
62 |
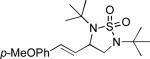
| 7 |
|
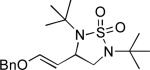
|
61 |
| 8 |
|
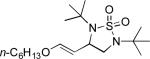
|
61 |
| 9[c] |
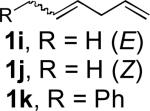
|
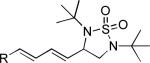
|
42 |
| 10[d] | 40 | ||
| 11[c] | (Z, E mixture, 6:1) | 66[f] |
All reactions were carried out with olefin (1.0 mmol), Pd2(dba)3 (0.050 mmol), tri-2-furylphosphine (0.30 mmol), and N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) (2.0 mmol) in benzene (0.25 mL) at 75 °C under argon for 10 h unless otherwise stated.
The reaction was carried out at 75 °C for 22 h.
The reaction was carried out at 65 °C for 6 h.
The reaction was carried out at 50 °C for 20 h.
Isolated yield.
7% yield of (1Z, 3E) isomer was also isolated.
