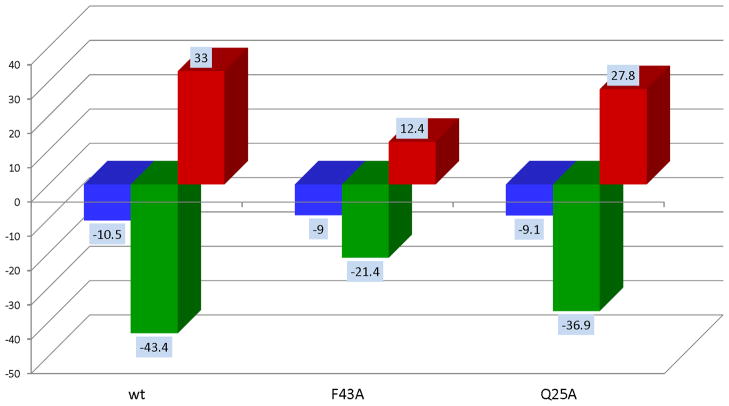
Figure 3.
Thermodynamic signatures for gp120 binding to wild type sCD4 and the Ala mutants F43A and Q25A. Mutations at specific locations may affect binding affinities (ΔG) differently indicating the contribution of specific residues to binding. Some mutations like F43A and Q25A reduce affinity by similar amounts but through different enthalpy/entropy combinations. F43A reduces the binding enthalpy (ΔH) by about half indicating that F43 is not only a hotspot for binding affinity but also for the allosteric structuring of gp120. Mutation of Q25, on the other hand lowers the affinity by a similar amount to that of F43A but with a much smaller reduction in binding enthalpy indicating that Q25 does not contribute significantly to allosteric structuring of gp120 even though it contributes significantly to binding. The magnitude of the allosteric structuring effect is also appreciated in the entropy changes. F43A does not cause as much structuring (smaller −TΔS) as wt or Q25A.