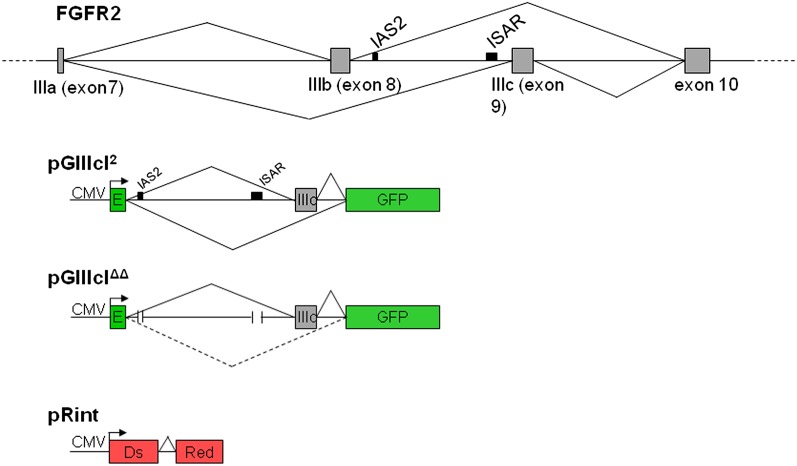
FIGURE 1.
Design strategy for the in vivo splicing reporters. The FGFR2 pre-mRNA is alternatively spliced within the last half of its third immunoglobulin-like domain to produce the IIIb (exon 8) or IIIc (exon 9) isoforms (top). To generate the pGIIIcI2 splicing reporter, the IIIc exon and flanking introns, including intronic activating sequence 2 (IAS2) and the intronic silencing and activating region (ISAR; black boxes), were inserted within the enhanced green fluorescent protein (EGFP) reading frame (green boxes). Skipping of IIIc (bottom lines) leads to fusion of the EGFP reading frame and expression of EGFP, while IIIc inclusion (top lines) interrupts the EGFP reading frame. Another reporter, pGIIIcIΔΔ, harbors deletions in IAS2 and ISAR, which abrogates cell-type-specific exon IIIc skipping (indicated by the dashed lines), resulting in almost complete inclusion of exon IIIc (solid lines). The pRint construct contains the DsRed open reading frame interrupted by a short adenoviral intron with consensus 5′ and 3′ splice sites, a branch point consensus sequence, and a polypyrimidine tract. This reporter controls for CMV-driven transcription and proper constitutive splicing at the ROSA26 locus in a specified cell type or tissue. Using the pRint control allows us to interrogate the splicing pattern of only those cells or tissue types that are expressing DsRed. If a cell or tissue is not expressing DsRed, we assume there is an interruption in transcription via CMV or constitutive splicing and do not interrogate GIIIc splicing regulation in these locations.