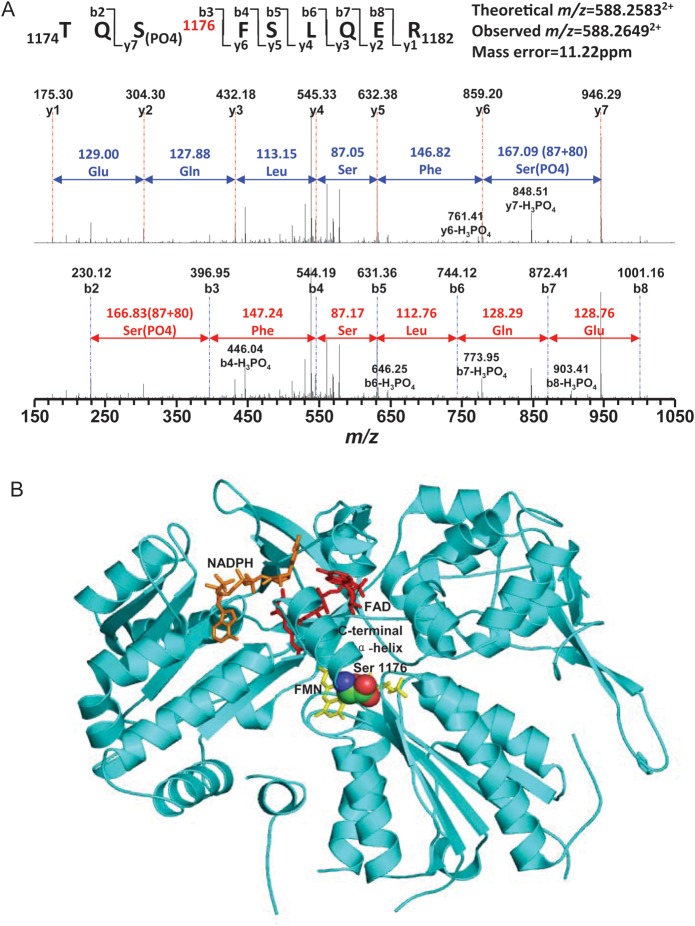
Figure 6.
Mass spectrometric identification of eNOS phosphorylation at Ser1176 in rat hearts subjected to IPC. (A) MS/MS spectrum of doubly protonated molecular ion, m/z = 588.26492+ of amino acids 1174–1182 of the eNOS C-terminal region. Rat eNOS was purified from IPC hearts by immunoprecipitation with eNOS antibody. The phosphorylation site of eNOS at Ser1176 in rat heart was determined from tryptic fragment 1174TQS(PO4)1176FSLQER1182. MW difference of 80 Da is seen between fragment ions y6 and y7 compared with the native fragment ions, which confirmed phosphorylation of Ser1176. The sequence-specific ions were labelled as y and b ions. (B) Molecular model of the eNOS reductase site with phosphorylation at Ser1176 showing its critical position in the C-terminal α-helix close to the critical FAD cofactor.