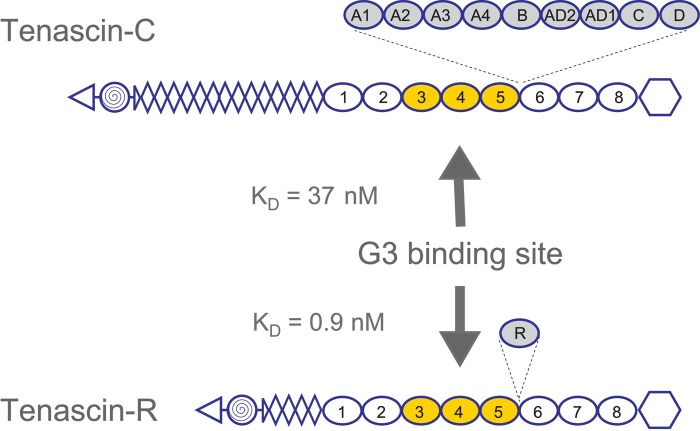
Figure 3.
Lectican C-type lectin domain (CLD) binding sites on tenascins. Lectican CLD binding sites on tenascin-R and tenascin-C were mapped to fibronectin type III repeats 3 to 5 using panels of overlapping recombinant fragments. Affinities, determined by BIAcore surface plasmon resonance experiments, are in the low nanomolar range (KD:values for the aggrecan CLD are shown). The use of bacterially expressed tenascin proteins showed that the interactions were carbohydrate independent. The domain organization of the tenascins is shown with triangles for N-termini, spiral-filled circles for multimerization domains, diamonds for epidermal growth factor (EGF)–like repeats, ovals for fibronectin type III repeats, and hexagons for fibrinogen globules. Alternatively spliced fibronectin type III repeats are shadowed and shown with their insertion sites marked by dashed lines.