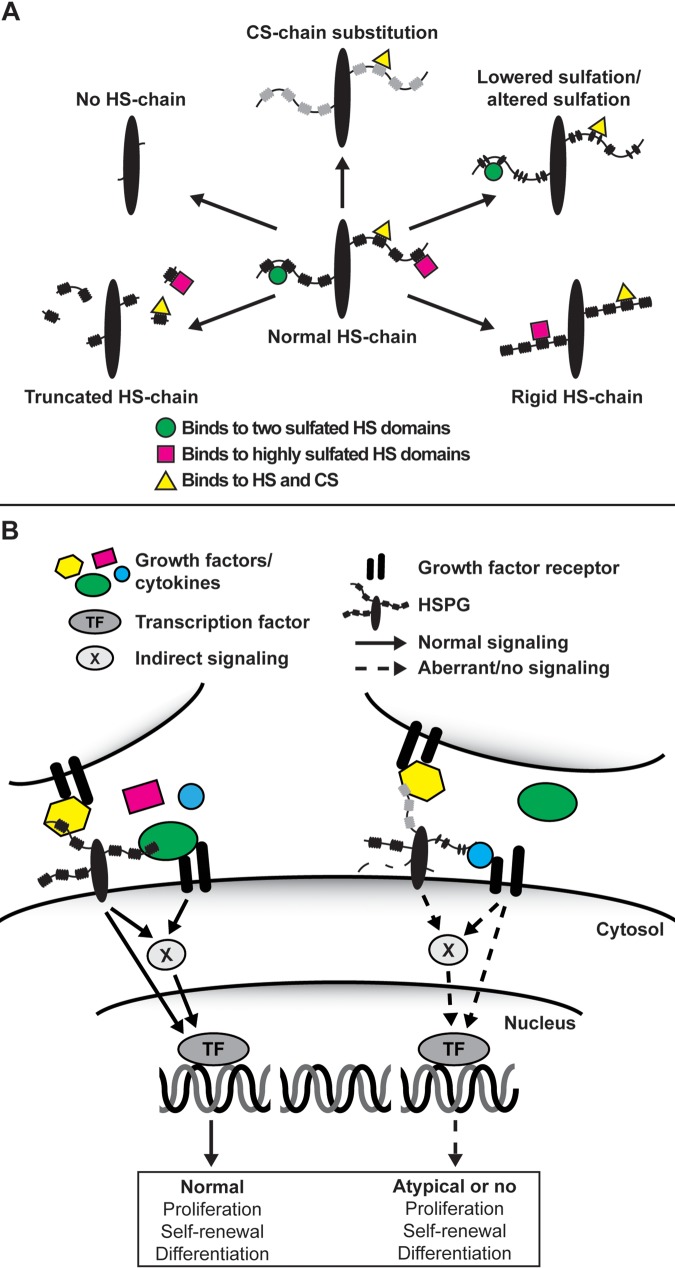
Figure 1.
(A) Hypothetical heparan sulfate (HS) chain structural consequences and effects on ligand interaction upon various mutations in the HS biosynthesis machinery. Depending on the enzyme being modified, various degrees of structural defects in the HS chain will occur ranging from a complete lack of HS chains (Ext1−/−) and exhibiting different degrees of sulfation (various HS sulfotransferase knockouts) to chain length (Hpse−/−) and HS chain rigidity (Glce−/−). The loss of HS in the Ext1−/− cells can also lead to a compensatory increase in chondroitin sulfate (CS) biosynthesis. (B) In wild-type embryonic stem (ES) cells (left), heparan sulfate proteoglycans (HSPGs) located on the same or an adjacent cell function as co-receptors for growth factors and their receptors or, upon ligand binding, induce downstream signal transduction on their own. Although not shown in the figure, HSPGs also facilitate cell adhesion to the extracellular matrix and, when secreted, are involved in the formation of the extracellular matrices that, for example, sequester growth factors and morphogens. However, in the various mutant ES cells (right), in which the HS synthesis and modification machinery have been targeted, an altered HS chain will change the extracellular information input received by the cell, subsequently affecting processes such as self-renewal capacity and pluripotency.