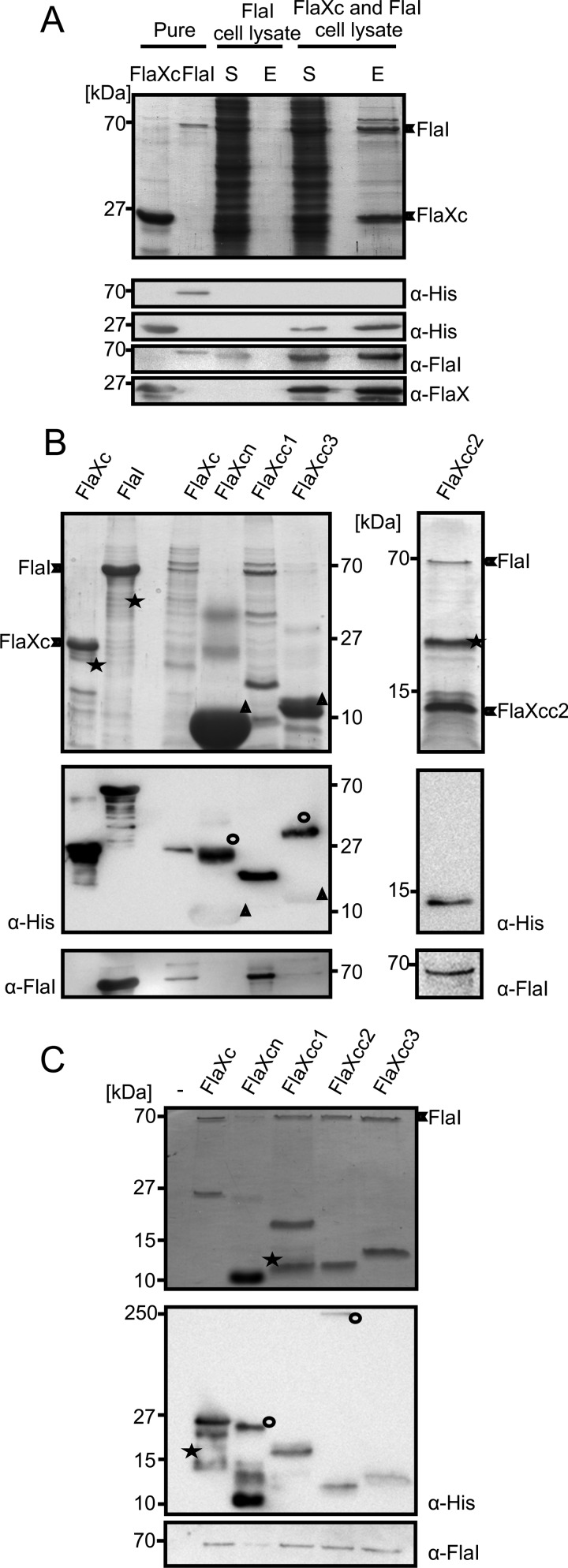
FIGURE 4.
FlaX and FlaI interact. A, cell lysates containing either untagged FlaI or FlaXc-His6 were mixed and FlaXc-His6 was isolated using Ni-NTA beads. Samples were analyzed on SDS-PAGE and immunoblotted with antibodies against the His6 tag, FlaI, and FlaX. Whereas untagged FlaI did not bind to the beads itself (“FlaI cell lysate”), it did co-elute with FlaXc-His6 (“FlaXc/FlaI cell lysate”). S, start material; E, elution fraction. His6-tagged FlaI was used in lane 2 to confirm the migration pattern of untagged FlaI. B, N-terminal His6-tagged FlaX truncates were co-expressed with untagged wt FlaI and Ni-NTA beads were used for pulldown analysis. The pulldown fraction was confirmed using SDS-PAGE (upper panels) and anti-His and anti-FlaI Western blot analysis (lower panels). ★, degraded protein. ○, higher oligomeric forms of the protein. Δ, monomer of the protein. FlaXcn and FlaXcc3 form SDS-resistant higher oligomers in a concentration-dependent manner and on Western blot analysis the higher oligomers provide more signal than the monomeric form, which is visible on the anti-His Western blot. The degradation product in the most right-hand panel reacted with the FlaI antibody. C, purified FlaX truncates were incubated with untagged FlaI as described in supplemental ”Experimental Procedures“ pulled down with Ni-NTA beads. Elution fractions were analyzed on SDS-PAGE (upper panel) and Western blot with either anti-His or anti-FlaI antibodies (lower panel). ★, degraded protein; ○, SDS-resistant oligomer.