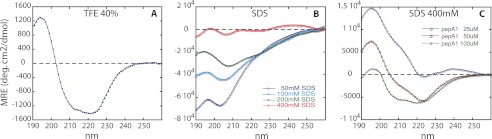
FIGURE 5.
Evolution of PepA1 as a function of SDS and peptide concentrations followed by circular dichroism. A, CD spectrum of PepA1 175 μm in 40% TFE, with the hallmark of the presence of a helix in its structure (positive peak at 195 nm and two negative peaks at 208 and 222 nm). B, PepA1 (10 μm) CD spectra in increasing concentrations of SDS above the CMC (the negative charges of SDS mimic those of the bacterial membranes). The helical content of PepA1 increases with the SDS/peptide ratio. C, PepA1 CD spectra recorded at a fixed SDS concentration (400 mm) with increasing PepA1 concentrations.