FIGURE 2.
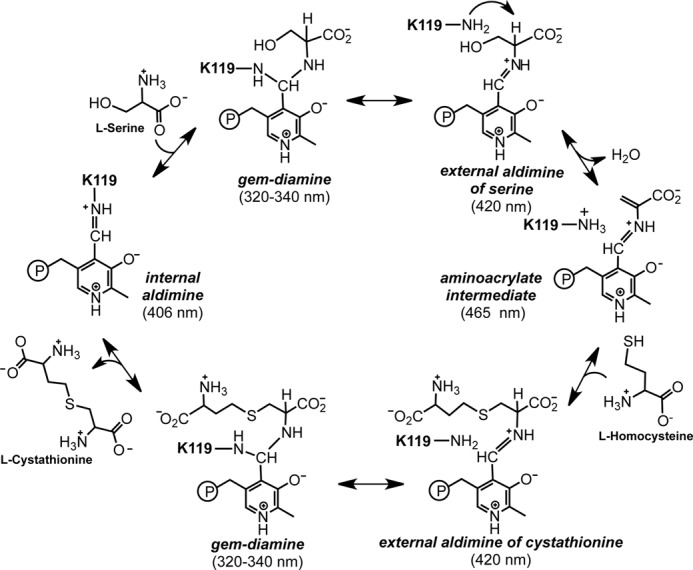
Proposed catalytic cycle of CBS. The resting enzyme exists as the internal aldimine with Lys-119 in human CBS involved in Schiff base linkage. Addition of serine (or cysteine) gives a gem-diamine intermediate, which collapses to the external aldimine of serine (or cysteine). Elimination of water (or H2S) leads to the aminoacrylate intermediate to which homocysteine (cysteine or water) adds to form the external aldimine of the product cystathionine (lanthionine or serine). The latter is released via the transient formation of a gem-diamine intermediate in a transchiffization reaction involving the active site lysine. Elimination of cystathionine from the active site completes the catalytic cycle and regenerates the resting enzyme. The numbers in parentheses represent the absorption maxima of the intermediates.
