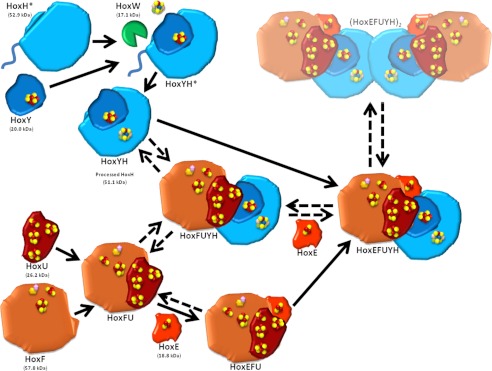
FIGURE 8.
Model for Hox hydrogenase association in Synechocystis sp. PCC 6803. Unprocessed HoxH (HoxH*; containing an additional 16-amino acid C terminus) binds HoxY (containing a [4F-4S] cluster) and undergoes assembly and insertion of its [NiFe] active site and cleavage of its C terminus by the protease HoxW, resulting in a hydrogenase subcomplex (HoxYH). Alternatively, HoxF (containing NAD and FMN binding sites and [2Fe-2S] and [4Fe-4S] clusters) associates with HoxU (containing one [2Fe-2S] and three [4Fe-4S] clusters) and HoxE (containing a [2Fe-2S] cluster), forming the diaphorase subcomplex (HoxEFU). The presence and formation of HoxFU and HoxFUYH subcomplexes may represent steps in assembly, but may also formally be due to a loss of HoxE during preparation/extraction strategies. Purification by Schmitz et al. resulted in detection of a dimer of the HoxEFUYH pentamer ((HoxEFUYH)2), which is depicted here despite our inability to detect this dimer in our preparations. We hypothesize that this dimerization may be through HoxH, allowing association of unprocessed HoxH with the full complex.