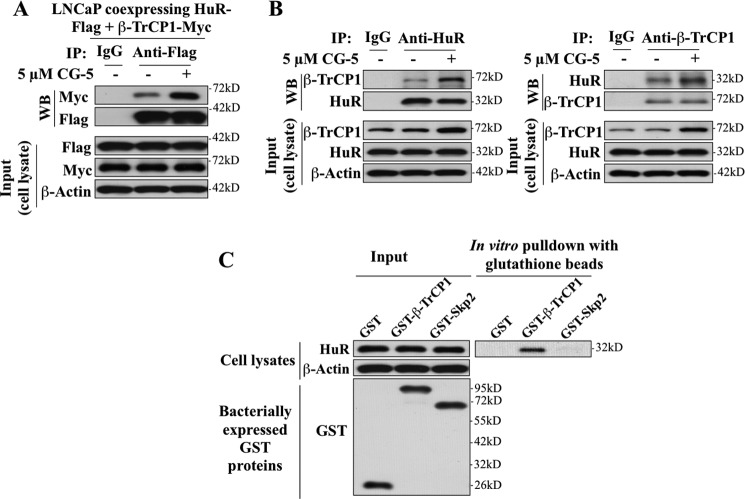
FIGURE 6.
Evidence that HuR physically interacts with β-TrCP1. A, coimmunoprecipitation analysis revealed the association of HuR with β-TrCP1 in response to CG-5 treatment. LNCaP cells ectopically expressing both FLAG-tagged HuR and Myc-tagged β-TrCP1 were treated with 5 μm CG-5 for 12 h, followed by cotreatment with 10 μm MG132 for an additional 12 h. Equal amounts of cell lysates were immunoprecipitated (IP) with anti-FLAG antibody and protein A/G-agarose followed by immunoblotting (WB) with anti-Myc and anti-FLAG antibodies. B, coimmunoprecipitation analysis showed the association of endogenous HuR and β-TrCP1 in response to CG-5 treatment. Cells were treated according to the aforementioned procedure. Equal amounts of cell lysates were immunoprecipitated with anti-HuR (left panel) and anti-β-TrCP1 (right panel) antibodies and protein A/G-agarose, followed by immunoblotting with anti-β-TrCP1 and anti-HuR antibodies, respectively. C, in vitro pull-down of HuR by bacterially expressed GST-β-TrCP1. Equal amounts of LNCaP cell lysates were incubated with recombinant GST, GST-β-TrCP1, or GST-Skp2 immobilized onto glutathione beads. The resulting complexes were washed, centrifuged, and subjected to immunoblotting analysis with HuR antibody (right panel). One-tenth volume of cell lysates were collected as input and probed with HuR and β-actin antibodies, and recombinant GST-fusion proteins were purified and probed with GST antibody (left panel).