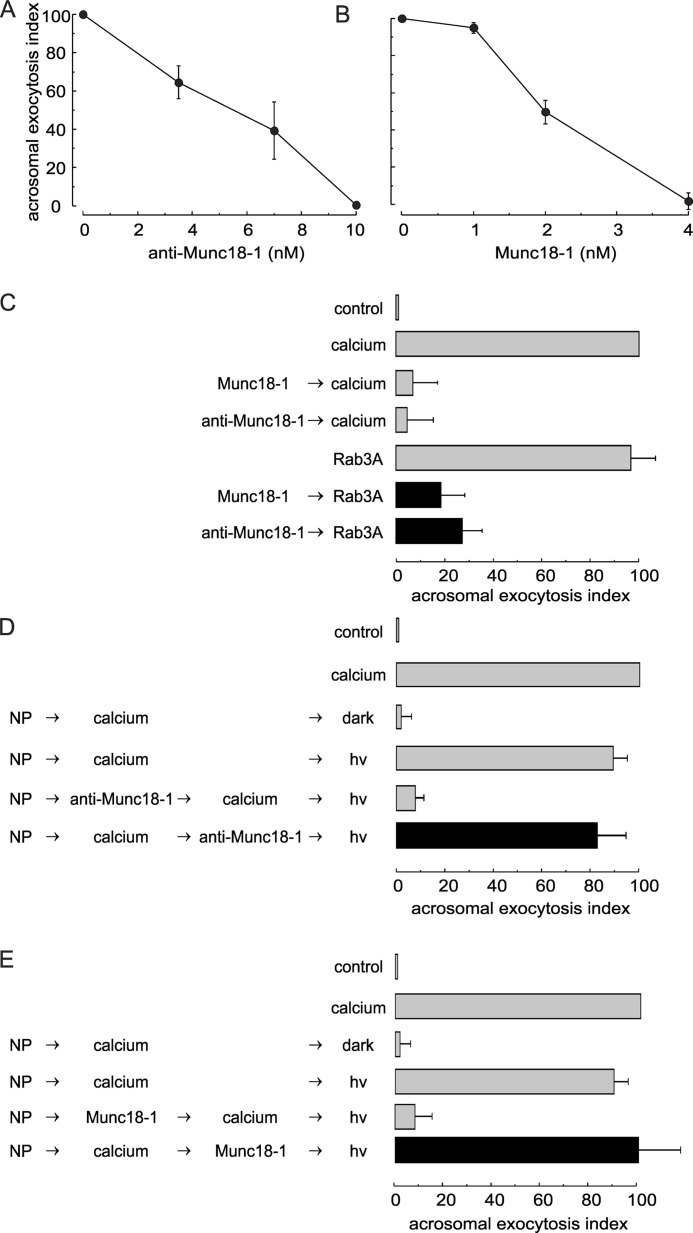
FIGURE 2.
Munc18-1 is essential for human sperm acrosomal exocytosis. A and B, SLO-permeabilized human sperm were treated for 15 min at 37 °C with increasing concentrations of anti-Munc18-1 antibody (A) or recombinant Munc18-1 (B). Exocytosis was initiated with 0.5 mm CaCl2, and cells were incubated for a further 15 min. The values represent mean ± S.E. of at least three independent experiments. C, permeabilized sperm were treated with 4 nm Munc18-1 (Munc18-1) or 10 nm anti-Munc18-1 antibody (anti-Munc18-1) before initiating acrosomal exocytosis with 300 nm prenylated and GTPγS-loaded Rab3A (Rab3A, black bars). Controls (gray bars) included the following: background exocytosis in the absence of any stimulation (control); exocytosis stimulated by 0.5 mm CaCl2 or 300 nm GTPγS-bound Rab3A, and the effect of 4 nm Munc18-1 or 10 nm anti-Munc18-1 on calcium-stimulated exocytosis. D and E, SLO-permeabilized sperm were loaded with 10 μm NP-EGTA-AM (NP) for 10 min at 37 °C to chelate intravesicular calcium. Next, acrosomal exocytosis was initiated by adding 0.5 mm CaCl2 (calcium), and samples were incubated for further 10 min at 37 °C. This protocol allows exocytosis to proceed up to the intra-acrosomal calcium-sensitive step. At this stage, sperm were treated for 10 min with 10 nm anti-Munc18-1 (anti-Munc18-1; D) or 4 nm Munc18-1 (Munc18-1; E). All these procedures were carried out in the dark. UV photolysis of the chelator was induced at the end of the incubation period (hv), and the samples were incubated for 5 min to promote exocytosis (black bars). Several controls were included (gray bars) as follows: background exocytosis in the absence of any stimulation (control); exocytosis stimulated by 0.5 mm CaCl2; inhibitory effect of NP-EGTA-AM in the dark and recovery upon illumination, and the inhibitory effect of anti-Munc18-1 and Munc18-1 when present throughout the experiment. For all panels, acrosomal exocytosis was evaluated using PSA-FITC, and data were normalized as described under “Experimental Procedures.” The values represent mean ± S.E. of at least three independent experiments. The anti-Munc18-1 antibody used for these experiments was from Synaptic Systems.