FIGURE 10.
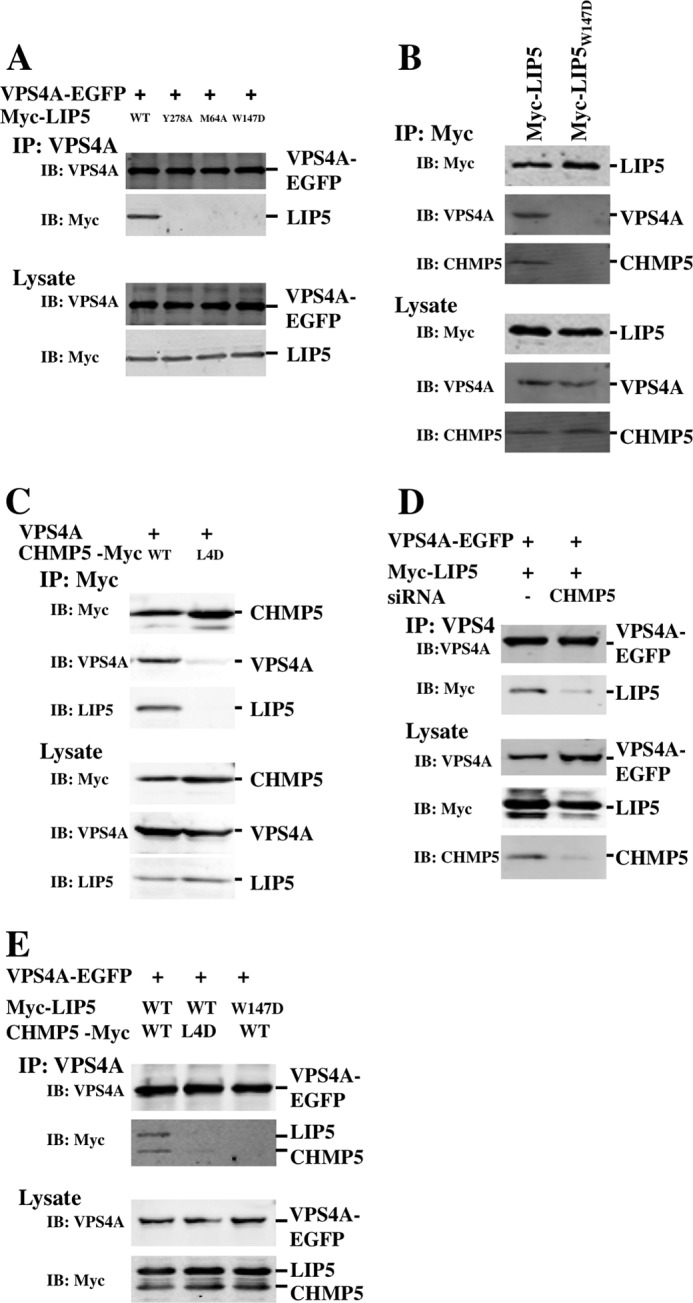
Immunoprecipitations of LIP5-ligand complexes. A, LIP5 co-immunoprecipitated with VPS4A (first lane), but the interaction was inhibited by LIP5 point mutations that blocked VPS4 (LIP5(Y278A); second lane), CHMP1B (LIP5(M64A); third lane) or CHMP5 (LIP5(W147D); fourth lane) binding. B, endogenous VPS4A and CHMP5 proteins co-immunoprecipitated with LIP5 (first lane) but not with the LIP5(W147D) mutant (second lane). C, VPS4A and endogenous LIP5 co-immunoprecipitated with CHMP5 (first lane) but not with a CHMP5 protein that contained leucine collar mutations (CHMP5(L163D,L167D,L170D,L174D (L4D); second lane). D, LIP5 co-immunoprecipitated with VPS4A (first lane), but the interaction was inhibited by siRNA depletion of the CHMP5 protein (second lane). E, LIP5 and CHMP5 co-immunoprecipitated with VPS4A (first lane), but the interactions were inhibited by a CHMP5 mutation (L163D,L167D,L170D,L174D) that inhibited LIP5 binding (second lane) or by a LIP5 mutation (W147D) that inhibited CHMP5 binding (third lane). IP, immunoprecipitation; IB, immunoblotting.
