Fig. 2.
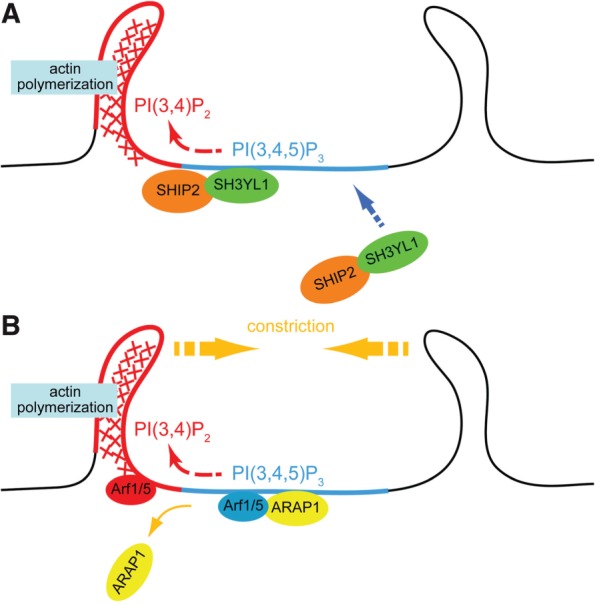
Models of CDR formation based on the phosphoinositide conversion from PI(3,4,5)P3 to PI(3,4)P2. (A) Upon stimulation with growth factors, PI3K is activated and produce PI(3,4,5)P3-rich area on the plasma membrane (blue). SH3YL1 is then recruited to the membrane via its SYLF domain that recognizes PI(3,4,5)P3. SH3YL1 simultaneously makes complex with a phosphoinositide 5-phosphatase SHIP2, resulting in a formation of PI(3,4)P2-enriched area (red) by dephosphorylation of PI(3,4,5)P3. Downstream target of PI(3,4)P2, such as Tapp1, is thought to be involved in actin polymerization. (B) ARAP1, which contains PH domains that collectively bind to PI(3,4,5)P3, is recruited to the plasma membrane and it then keeps Arf1/5 in a GDP-bound state (blue). When the phosphoinositide conversion from PI(3,4,5)P3 to PI(3,4)P2 takes place, ARAP1 detaches from the plasma membrane, allowing the activation of Arf1/5 into its GTP-bound form (red).
