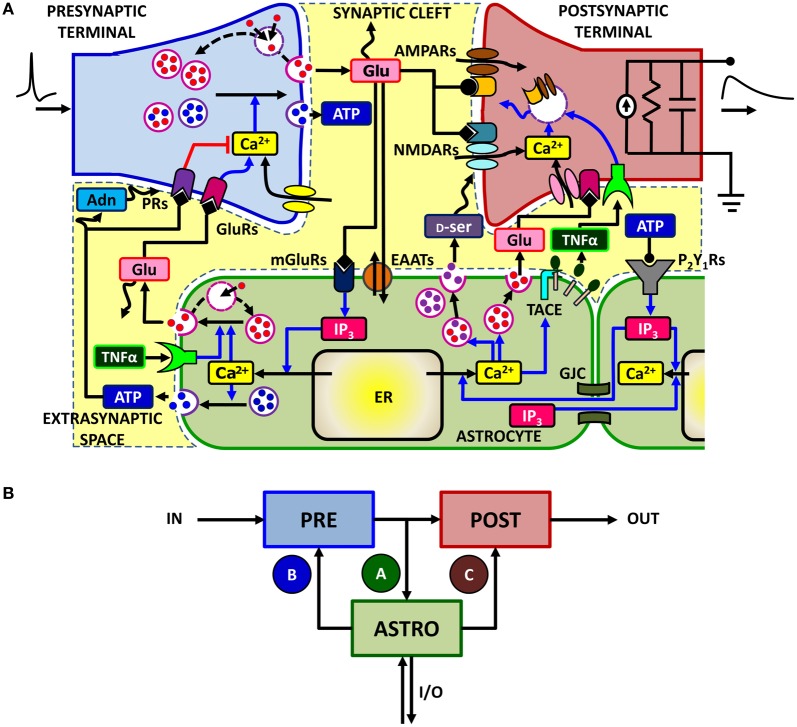
Figure 1.
The signaling network of astrocyte-synapse interactions. (A) A simplified scheme of the different signaling pathways between synaptic terminals and astrocytes for the case of excitatory synapses in the hippocampus (see text for a detailed description). Action potentials arriving at the presynaptic terminal trigger release of glutamate, which can spill over from the synaptic cleft. Perisynaptic astrocytes take up glutamate using their plasma membrane transporters (EAATs) while glutamate, by acting on astrocytic metabotropic receptors (mGluRs), triggers Ca2+ signaling in the astrocyte. This signaling pathway includes production of IP3 and causes an increase of cytosolic Ca2+ due to efflux of this ion from the endoplasmic reticulum (ER). At some synapses, such as in the dentate gyrus, the same Ca2+ signaling pathway could also be mediated by astrocytic purinergic P2Y1 receptors, likely activated by synaptically-released ATP (see text for details). Astrocytic Ca2+ excitability can in turn lead to exocytotic release of several neuroactive substances (or “gliotransmitters”) such as glutamate (Glu), D-serine (D-ser) or ATP which can target specific receptors on pre- and post-synaptic terminals and differentially modulate synaptic transmission. Glutamate acting on presynaptic GluRs could enhance synaptic release, whereas ATP and its derivate adenosine (Adn) could depress it (red path) through presynaptic purinergic receptors (PRs). On the postsynaptic spines [depicted here by a standard RC circuit (Ermentrout and Terman, 2010)], the ensuing effect of gliotransmitters could substantially modify postsynaptic currents by enhancing activation of NMDA receptors (D-serine) or by altering expressions of AMPA receptors therein. Astrocytes could also release TNFα by Ca2+-dependent activation of the matrix metalloprotease TNFα-converting enzyme (TACE), while extracellular TNFα could in turn regulate glutamate release from the astrocyte as well as postsynaptic AMPAR expression. Moreover astrocytic Ca2+ could also propagate across different regions of the same cell or to other neighboring astrocytes by intracellular IP3 diffusion through gap junction channels (GJCs) or via extracellular ATP-dependent pathways, extending gliotransmission to some distal sites away from the considered synapse. For clarity both endocannabinoid-mediated Ca2+ signaling (Navarrete and Araque, 2008), retrograde activation of presynaptic glutamate receptors (Navarrete and Araque, 2010), regulation of postsynaptic NMDARs by presynaptic adenosine receptors (Deng et al., 2011), and the possibility for astrocyte-derived adenosine to enhance synaptic release (Panatier et al., 2011) are not included in this scheme. (B) Equivalent modeling scheme for astrocyte-synapse interactions. The astrocyte (ASTRO) constitutes a third active element of the tripartite synapse in addition to the presynaptic (PRE) and postsynaptic (POST) terminals. In its presence, the flow of input (IN) signals to the output (OUT) is no more unidirectional but presynaptically released neurotransmitter can affect astrocyte function through the interaction pathway A. In turn, the astrocyte can regulate both synaptic terminals via pathways B and C. In addition, the astrocyte could receive additional inputs from or send output to remote synapses in a heterosynaptic fashion (I/O).